| |
Intermittent therapy for the treatment of chronic HIV infection, EDITORIAL REVIEW
|
| |
| |
AIDS: Volume 21(2) 11 January 2007 p 123-134
Ananworanich, Jintanata,b,c; Hirschel, Bernardd
From the aHIV Netherlands Australia Thailand Research Collaboration (HIV-NAT), Bangkok, Thailand
bJohn A. Burns School of Medicine, University of Hawaii, Manoa, Honolulu, USA
cSouth East Asia Research Collaboration with Hawaii (SEARCH), Bangkok, Thailand
dGeneva University Hospital, Geneva, Switzerland.
Received 3 April, 2006
Accepted 16 June, 2006
AUTHOR CONCLUSION
Perspective on the future of scheduled treatment interruption
SMART raises many burning questions, without clear answers at the present time. Among the most important of these is how to explain the differences between SMART and other STI studies such as Staccato. Had the event rates of SMART applied to Staccato, between 15 and 20 events would have occurred, whereas just one event was observed. The most important differences between the two studies are the CD4 cell count at which treatment was started again (250 cells/_l in SMART and 350 cells/_l in Staccato), the length of time on HAART before the study (72 months in SMART and 15 months in Staccato), the length of interruption (18 months in SMART and 18 weeks in Staccato) and the age of the patients (median 46 years in SMART and 35 years in Staccato) [5,15]. It is possible, but far from proven, that these factors, particularly the longer duration of previous treatment and of interruption and the lower CD4 cell count threshold for restarting HAART in SMART, contributed to the divergent outcomes.
The SMART study was large and well executed, with results that clearly show that STI increase the risk of AIDS-defining OI and death; moreover, the hoped-for benefits in terms of decreased cardiovascular and hepatic morbidity were not seen. To put it in a nutshell, in our opinion, the results of SMART suggest: 'Once you have started HAART, you never can stop again'.
So what is the future for STI? Although quality of life is hard to quantify, many patients may feel that their 'quality of life' is improved by time off drugs, and the one in 50-100 risk of something bad happening is worth taking. Moreover, the need to interrupt therapy for drug toxicities will remain an important issue in HIV treatment. That is why efforts to make STI safer will continue, perhaps using higher CD4 cell count start and stop criteria. Given the SMART results, however, it is unlikely that another large prospective study with clinical endpoints will be funded. Preliminary data on fixed cycle STI with at least 8 weeks on HAART are encouraging, and may provide an option of a convenient STI with a 50% reduction in drug use and simplified monitoring. In the SMART study the Kaplan-Meier curves of event-free survival only diverge after 6 months, also suggesting that shorter STI might be safer.
Introduction
HAART has changed HIV infection from a life-threatening into a chronic condition, but the perspective of never ending HAART once it is started is daunting to many patients. As a cure for HIV infection is currently not in sight, many patients, who are looking to their physicians for advice on whether to stop HAART. New drugs and combinations with better safety and tolerability profiles have become available [1], but there will still be patients who experience toxicity [1-3]; therefore, being able to take a rest from HAART may be beneficial [3]. Worldwide, the cost of treating millions with HIV burdens fragile economies; intermittent treatment promises relief. Many scheduled treatment interruption (STI) studies have shown between 40 and 60% drug savings with STI [4-7]. For example, in Thailand, using the least expensive generic fixed dose combination of stavudine/lamivudine/nevirapine, which is US$27 per month, the yearly cost of treating 500 000 HIV-positive individuals would decrease from US$162 million to US$81 million if there is a 50% drug saving. Moreover, STI may provide the possibility that a potent and inexpensive drug such as stavudine may still be used without many side-effects, whereas continuous use would necessitate its replacement by a less toxic, but mostly more expensive, alternative. However, these potential benefits have to be weighed against the potential risks, which include the development of HIV-related illnesses, acute retroviral syndrome, thrombocytopenia, resistance and the transmission of HIV infection.
Most individuals with HIV are diagnosed and treated during the chronic HIV infection phase. In those who have a good response to HAART and are able to maintain high CD4 cell counts and low viral loads (VL) for a period of time, STI have been explored with fixed time strategies (such as one week on-one week off, or 8 weeks on-8 weeks off) or with varying off times depending usually on the CD4 cell count. The goal here is to improve quality of life and HAART toxicity, and lower costs while maintaining patients' good health and a safe level of CD4 cells. Initial studies have been small and uncontrolled: later studies have enrolled several hundred patients and used a control group of continuously treated patients. On the whole, results have been quite encouraging [4,8-13], although a meta-analysis of STI studies published from January 1996 to March 2005 concluded that evidence to support STI was inconclusive [14]. In January 2006, the largest STI study that had recruited over 5000 patients, the Strategy for Management of Antiretroviral Therapy (SMART), showed that an STI strategy using CD4 cell counts as a guide led to an increase in HIV and non-HIV-related serious illnesses. As a result, recruitment was stopped prematurely [15].
In this article, we present the rationale for STI, review data on STI in chronic HIV infection, consider the effects of STI on the selection of resistance mutations, and finally provide a perspective on the future of STI.
CD4 cell counts after stopping treatment
CD4 cell counts decline when HAART is stopped. The most dramatic drop occurs within the first 4 weeks, with an average decline of 30 cells per week, followed by a more gradual drop of 3 cells per week (Fig. 1) [16]. The VL usually becomes detectable 7 days after stopping and peaks at approximately 4 weeks [9]. CD4 cell counts tend to drop faster in patients who have low pre-antiretroviral CD4 cell counts, high CD4 cell counts before stopping and high VL at the time of peak VL rebound [11,16,17].
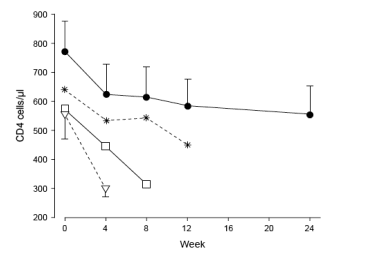
Fig. 1. Biphasic CD4 cell count decline after scheduled treatment interruption. The error bars show standard errors of the mean for scheduled treatment interruptions (STI) lasting 4 and 24 weeks. They have been deleted for STI lasting 8 and 12 weeks so as not to overload the figure. Data are from
patients enrolled in the Staccato and the Swiss Spanish Intermittent Treatment Trial studies showing CD4 cell declines in patients who were able to stop HAART for different lengths of time until they reached the CD4 cell count of less than
350 cells/ml restart criteria. It shows that patients with lower baseline CD4 cell counts reached the CD4 cell count restart criteria (< 350 cells/ml) before week 24. Regardless of the STI time, the CD4 cell count dropped most dramatically during the first 4 weeks, after which a more gradual drop was seen
[19]. (black dot) STI >24 weeks (112); (star) STI 12 weeks (19); (box) STI
8 weeks (43); STI (triangle) 4 weeks (24).
Scheduled treatment interruption studies in chronic HIV infection using CD4 cell counts as a guide
Stopping and starting treatment based on the CD4 cell count makes sense as it is the best predictor of HIV disease progression. This is also a strategy that is tailored to each individual's immune status providing an opportunity for some to be off treatment for a long time, particularly those who have a high baseline CD4 cell count. The different randomized CD4 cell count-guided (referred to hereafter as CD4-guided) studies in VL-suppressed patients are summarized in Table 1. Because of space constraints, studies that are non-randomized or use non-standard HAART are not described [12,13,18-21].
The BASTA study randomly assigned 69 patients with CD4 cell counts greater than 800 cells/μl to either continuous or CD4 cell count-guided HAART with a CD4 cell count restart criteria of less than 400 cells/μl. They found that the CD4 arm was safe and most CD4 arm patients could stop for at least one year. After 64 weeks, 72% had CD4 cell counts greater than 400 cells/μl [11].
The Tibet study randomly assigned 201 patients with CD4 cell counts greater than 500 cells/_l and VL less than 50 copies/ml to CD4 cell count-guided HAART (stopping at CD4 cell counts ≥ 500 cells/_l and starting at CD4 cell counts < 350 cells/_l compared with continuous HAART). At 96 weeks, no one had AIDS-defining illness. The CD4 arm had more adverse events related to the antiretroviral syndrome after stopping treatment but had lower adverse events from antiretroviral drugs. The drug exposure was 68% less in the CD4-guided arm compared with the continuous arm [22].
The ACTG 5102 evaluated the use of IL-2 in combination with HAART to raise the CD4 cell count before stopping therapy. Almost all patients were able to stop for the 48-week duration and the HAART plus IL-2 arm had a higher CD4 cell count [23].
The HIV-NAT 001.4 study was a pilot study to Staccato but was performed in a different patient population: HIV-NAT 001.4 patients were dual nucleoside reverse transcriptase inhibitor (NRTI)-pretreated compared with patients newly treated with HAART in Staccato; nevertheless, the results were similar to those of Staccato [4].
Staccato evaluated a CD4 stop/start threshold of 350 cells/_l in 284 patients who had baseline CD4 cell counts greater than 350 cells/_l in comparison with 146 continuous HAART patients. After almost 2 years, there was no difference in AIDS or death [one death or 0.4 per 100 person-years (100PY) in the continuous versus one death or 0.2 per 100PY in the STI arms] and the VL responses to HAART retreatment were the same between the arms, with approximately 90% having VL of less than 50 copies/ml. The CD4 arm, however, had more minor HIV-related illnesses including oral and vaginal candidiasis (2.28/100PY in the CD4 versus 0.34/100PY in the continuous arms, P = 0.04). The CD4 arm had lower CD4 cell counts (median 374 cells/_l in the CD4 arm versus 601 cells/_l in the continuous arm), but the CD4 cell count rose rapidly after 12 weeks of HAART and there was a significant antiretroviral drug saving. In addition, the patients in the CD4 arm felt they had less lipodystrophy and the cholesterol level was lower, and more patients in the continuous arm had antiretroviral drug-related neuropathy and diarrhea [5].
Trivacan and SMART both enrolled patients with similar criteria as Staccato, baseline CD4 cell counts greater than 350 cells/_l and VL below the limits of detection (the latter for all patients in Trivacan and 72% in SMART), but the start/stop CD4 cell count criteria were different. Both of the studies stopped HAART at CD4 cell counts greater than 350 cells/_l and restarted at CD4 cell counts of less than 250 cells/_l, whereas in Staccato, treatment started again when the CD4 cell count declined below 350 cells/_l. In Trivacan and SMART, there was a significantly higher incidence of serious illnesses in the CD4-guided arm. In the Trivacan study, World Health Organization stage 3 and 4 illnesses were found in 17.6/100PY in the CD4 arm compared with 6.7/100PY in the continuous arm (P = 0.001). The most common serious illness was invasive bacteremia, which is not usually considered an HIV-related disease. Oral and vaginal candidiasis was also more common in the CD4 arm [7]. In the SMART study, the number of opportunistic infections (OI)/death events were 3.3/100PY in the STI arm compared with 1.3/100PY in the continuous arm (P < 0.0001). The excess risk of the CD4-guided arm was evident in all subcategories: death (1.5/100PY in CD4 arm versus 0.8/100PY in the continuous arm, P = 0.007), serious OI (0.4/100PY in the CD4 arm versus 0.1/100PY in the continuous arm, P = 0.01) and non-serious OI (1.7/100PY in the CD4 arm versus 0.5/100PY in the continuous arm, P < 0.001). The most common cause of death was cancer, and only 7% of death was due to OI. Esophageal candidiasis was the most common non-fatal OI in both arms. For non-OI events, major cardiovascular diseases and metabolic events were 1.8/100PY in the CD4 arm compared with 1.0/100PY in the continuous arm (P = 0.01) with events in the subgroups as follow: fatal or non-fatal cardiovascular diseases (1.3/100PY in the CD4 arm versus 0.8/100PY in the continuous arm, P = 0.06), fatal or non-fatal renal disease (0.2/100PY in the CD4 arm versus 0.1/100PY in the continuous arm, P = 0.05) and fatal or non-fatal liver disease (0.3/100PY in the CD4 arm versus 0.2/100PY in the continuous arm, P = 0.46). The grade 4 severe toxicities according to the Division of AIDS, National Institutes of Allergy and Infectious Diseases grading system were not different in the two arms (4.8/100PY in the CD4 arm versus 4.1/100PY in the continuous arm, P = 0.20; the SMART Study Group, in preparation).
SMART patients had up to 4 years of follow-up, with a mean follow-up time of 16 months. Patients were able to stop for a long period: the median first period off HAART was 18 months (interquartile range 5.8-43.5 months). The hazard ratio of both OI/non-OI events appear to increase in the CD4 arm after 8 months in the study. Most patients stopped only once, with approximately 12 and 2% stopping two or three times. With the HAART re-initiation CD4 cell count criteria of less than 250 cells/_l, the time patients spent at CD4 cell counts below 200 cells/_l was only 3% of the total study time. The median CD4 cell count at re-initiation was 234 cells/_l. After HAART retreatment, the response was good, with a median time to VL suppression below 400 of 3.1 months and a CD4 cell increase of 164 cells by 8 months. On average, the CD4 arm had 206 less CD4 cells/_l than the continuous arm. The time on HAART was much less in the CD4 arm (33%) compared with the continuous arm (94%).
In SMART, the following factors did not significantly affect the number of OI/non-OI deaths: sex, CD4 cell nadir, duration of previous HAART and baseline antiretroviral status (naive, experienced, on/off antiretroviral drugs). Older patients had a higher risk of AIDS/death events in both treatment groups. Being black increased the relative risk of events, comparing CD4-guided to continuous treatment. As expected, the superiority of the continuous suppression arm was mainly apparent in those patients whose viremia was undetectable at baseline. In the patients with non-suppressed viremia, the continuous and CD4-guided arms had similar outcomes: Non-suppressed viremia is evidence for ineffective treatment; whether such ineffective treatment is given continuously or intermittently doesn't matter.
The analysis of SMART was based on the 'latest' CD4 cell counts and VL results. These are values, before and closest, to the OI/non-OI endpoint events. As expected, having a lower latest CD4 cell count and higher latest VL puts one at more risk of both OI (fatal and non-fatal) and non-OI deaths for both the CD4 and continuous arms. Those who developed OI had a latest CD4 cell count at least 75-100 cells lower than the whole group for both arms. The latest CD4 cell count before OI events tended to be lower than those before non-OI events in both arms. What is surprising is that the risk of both OI and non-OI events is statistically higher in the CD4 arm than the continuous arm only when the latest CD4 cell counts exceeds 350 cells/_l.
Regarding the VL, the risk of OI (fatal and non-fatal) but not the non-OI deaths in both arms seems to be highest when the latest VL is greater than 50 000 copies/ml. But surprising again, the relative risk of both OI and non-OI deaths is higher in the CD4 arm compared with the continuous arm only when the latest VL is 400 copies/ml or less [15].
What does this all mean? The typical SMART patient had a high baseline CD4 cell count (the median pre-STI CD4 cell count in SMART was 597 cells/_l), a potentially large CD4 cell count drop to below 250 cells/_l and exposure to high VL over a long stop time (median time off after the first interruption in SMART was 18 months). In contrast, the typical STACCATO patient had lower CD4 cells at baseline, and the median time to starting treatment again was only 18 weeks. Do the OI and non-OI events occur while the patients are still off HAART, or do they occur after HAART has been restarted during the CD4 cell increase and VL decline period? This may be similar to an immune reconstitution syndrome model in which the inflammation occurs when there is a rapid decline in VL and a rise in the CD4 cell count, with an increased incidence of OI shortly after starting HAART. However, immune reconstitution syndrome occurs in patients who are more severely immune suppressed than those who participate in STI studies and indeed, analyses of SMART data presented in meetings are not in favor of this hypothesis.
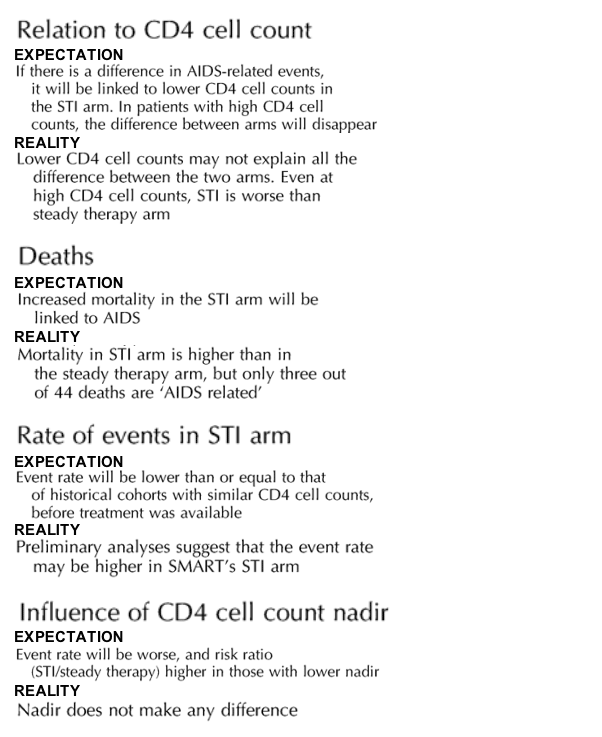
The results of SMART confounded expectations, as shown in Table 2. (See Table 2 at end of article)
In summary, of the six randomized studies, vour (BASTA, ACTG 5102, HIV-NAT 001.4 and Staccato) used a CD4 cell count restart threshold of 350-400 cells/_l and did not see increased serious morbidity, and two studies (Trivacan and SMART) used a CD4 cell count restart threshold of 250 cells/_l and saw an increase in serious morbidity. This suggests that the CD4 cell count restart threshold is important; however, all the studies except SMART did not have the statistical power to find a difference in serious morbidity. Therefore, at this time, it is unclear whether 'safe' CD4 cell count stop/start thresholds (thresholds at which the difference between an STI and continuous treatment strategies would disappear) can be defined.
Other factors than the CD4 cell counts may be important. Compared with SMART, Staccato enrolled patients with lower CD4 cell counts. In the CD4 cell count-guided arm, HAART was restarted at 350 CD4 cells compared with 250 in SMART. Consequently, STI were much shorter in Staccato (median of 18 weeks compared with 18 months in SMART). Another study (Windows) mentioned below used STI with a fixed length of 8 weeks. Both Staccato and Windows showed no excess in OI/death events in the STI arms. Had the event rates of SMART applied to Staccato, 16 such events would have been expected in the CD4 arm, whereas only one was observed. On the basis of these data, one may speculate that the length of treatment interruptions matters; long, but not short STI increase the risk of OI/death.
It will also be important to decide globally whether a strategy that has the potential of reducing drug costs and toxicity and improving quality of life should be abandoned altogether as a result of the 2.0/100PY difference in the AIDS/death rate compared with continuous HAART in SMART. Further information from SMART on the non-HIV-related illness occurrence such as cardiovascular complications in the CD4 arm and the CD4 dynamics in those who developed disease progression will help in understanding how best to proceed.
Scheduled treatment interruption studies in chronic HIV infection using fixed time intervals
Fixed time STIs aim to maintain a high CD4 cell count with a low risk of disease progression, and to achieve significant drug savings using a simple predictable strategy that a patient could easily follow. The different randomized studies in virally suppressed patients are shown in Table 3.
Dybul et al. [8] used a week on-week off schedule and showed that eight out of eight patients were able to maintain their VL at less than 50 copies/ml at the end of the week off for 68 weeks.
However, the HIV-NAT 001.4 and the Staccato study, both randomized studies comparing one week on-one week off with continuous and CD4 cell count-guided HAART, showed virological failure rates of 46 and 53%, respectively, leading to premature termination of that arm [4,24]. Fortunately, no significant resistance mutations were found in patients who failed, and when retreated with continuous HAART using the same regimen the VL was resuppressed.
The Five Days On, Two Days Off (FOTO) study, aim to give patients the weekend off of drugs, enrolled 30 subjects with CD4 > 200 cells/mm3 and undetectable VL who were on different HAART: 10 on NVP-based, 10 on EFV-based and 10 on PI-based. All those taking EFV had VL < 50 copies while 1 or 2 of those in the other arms had VL rebound. This may be due to the long half life of EFV: EFV level was maintained at the end of 2-day interruption [25].
Another fixed time strategy that had to be prematurely terminated because of an increased risk of resistance mutations to lamivudine and non-nucleoside reverse transcriptase inhibitor (NNRTI) used an 8 weeks on-4 weeks off schedule. After the enrollment of 56 patients, five out of 26 in the STI arm had resistance mutations. Most of the failing patients were treated with suboptimal therapy before HAART and may have had pre-existing mutations that probably contributed to the resistance seen after STI [26].
Window ANRS 106 looked at an 8 week-on schedule but with an 8-week off time instead of 4 weeks. The Window study enrolled 403 adults with a nadir CD4 cell count greater than 100 cells/_l, VL less than 200 copies/ml and CD4 cell count of 450 cells/_l or greater for 6 months or more. Intention to treat analysis showed no significant difference in clinical outcome and in the percentage of patients with CD4 cell counts less than 300 cells/_l at 96 weeks: 3.6% in STI compared with 1.5% in the continuous arms. The percentage with VL of 400 copies/ml or less was lower in the STI arm: 81 compared with 90% in the continuous arm (P = 0.02), but the incidence of virological failure and resistance were not different. There was also a 52.5% drug savings [6].
Papasavvas et al. [27] reported a two-phase study to evaluate whether repeated short interruptions before a long stop can delay the time to a VL greater than 5000 copies/ml. During the first phase, 42 patients either underwent three step-wise fixed interruptions of 2, 4 and 6 weeks, followed by HAART for up to 20 weeks until the VL was less than 50 copies/ml or continuous HAART for 40 weeks. In phase 2, both groups stopped HAART. The time to a VL of greater than 5000 copies/ml was approximately one month in both groups. Treatment failure was more common in the continuous arm; however, more resistance mutations during VL rebound were documented in the repeated interruption arm, but almost all patients resuppressed their VL after resuming the same regimen.
ISS-PART also evaluated a step-wise fixed time strategy with five STI of one, one, 2, 2 and 3 months' duration, each followed by 3 months of therapy, compared with continuous HAART. The median loss of CD4 cell to end of follow-up was 26 cells in the STI group. At 24 months, more than 90% in both arms had VL of less than 400 copies, and time to failure (defined as VL > 400 copies/ml after 12 weeks of treatment) were not different. Resistance mutations were found in 38 out of 136 STI patients, corresponding to a cumulative risk of resistance at 24 months of 30% [28]. These were supposed to be patients on their first regimen without evidence of resistance; however, many had resistance mutations in their baseline DNA and subsequently failed. In summary, the study did not demonstrate any benefit from a complicated intervention with increasing lengths of STI.
The Development of Anti-Retroviral Therapy in Africa (DART) trial is an open, randomized trial to evaluate HIV clinical disease progression with simplified antiretroviral therapy in Africa by studying two strategies: clinical monitoring alone compared with laboratory plus clinical monitoring, and STI (12 weeks on-12 weeks off) compared with continuous HAART. The trial has enrolled 3300 patients in Uganda and Zimbabwe. Pilot data of 799 patients followed up to 40 weeks showed that the STI arm had more HIV-related disease progression (8.6/100PY) compared with the continuous arm (2.0/100PY). Although most of the events were esophageal candidiasis, which were non-life threatening and did not require hospitalization, these were considered clinically important; therefore, the STI arm patients resumed continuous HAART as of March 2006. DART concluded that the 12 weeks on-12 weeks off strategy cannot be recommended in patients who have had a relatively short therapy time before STI and had low CD4 cell counts and many illnesses before HAART, such as those who participated in the trial [29].
Resistance in scheduled treatment interruption studies
After treatment is stopped, drug concentrations decrease at variable speeds. NRTI and protease inhibitors (PI) have shorter half-lives than NNRTI. A study in HIV-negative Dutch women showed that after a single dose of nevirapine, the median time to reach an undetectable level was 17 days (range 10 to > 21 days) in plasma and 14 days (range 7-21 days) in saliva [30]. Lower efavirenz clearance is associated with the cytochrome P450 2B6 gene polymorphism, which is common in African Americans; it could therefore take more than 14 days for efavirenz to be cleared [31]. During the tail end of these drug concentrations, HIV starts to multiply, potentially setting the stage for the selection of drug-resistant variants. When pregnant women receive a single dose of nevirapine to prevent mother-to-child transmission of HIV, resistance mutations occur commonly [32,33]. This is the reason why in most STI studies investigators stopped NNRTI between 3 and 14 days before NRTI in an attempt to reduce the risk of resistance [5,15,28]. The selection of resistance mutations is particularly likely if archived mutations exist in proviral DNA before STI [20,28,34-36]. However, there is evidence that new mutations are sometimes selected during STI, and it appears that the risk may be highest with lamivudine-based regimens and lowest with boosted-PI regimens.
In the study by Dybul et al. [26], none of the 26 patients randomly assigned to the continuous arm but five out of 26 of the 8 weeks on-4weeks off arm had resistance. This cohort was mainly treated with PI, but the NNRTI-treated patients were at more risk as three out of eight efavirenz-treated patients had resistance: two with K103N and one with M184V. The other two resistant patients were on PI and had resistance to NRTI: one with M184V and one with T215Y. It is important to note that most resistant patients had a history of previous suboptimal antiretroviral treatment (see Table 4).
In the Swiss Spanish Intermittent Treatment Trial (SSITT) [9], patients had four cycles of 2 weeks off-8 weeks on, and 17% of the 87 patients whose VL was above 50 copies/ml after 8 weeks of retreatment had new mutations, mostly M184V/I.
In the Tibet study [22], one CD4 arm patient and nine continuous arm patients had virological failure. Eighty-seven CD4 arm patients had genotyping performed on a viral rebound sample after interruption, and 31 had NRTI/NNRTI mutations. Of the nine virological failure patients in the continuous arm, five samples were amplified and three had mutations. NRTI mutations were mostly archived, but many of the NNRTI mutations were newly acquired.
In HIV-NAT 001.4 [37], using non-lamivudine, ritonavir-boosted PI regimens, there was no selection of major mutations in the CD4 arm, even though many patients had a history of treatment failure on previous suboptimal regimens.
Staccato had 22 out of 146 continuous arm and 10 out of 284 CD4 arm patients with at least one VL greater than 500 copies/ml after at least 12 weeks on HAART [40]. Of these, only one STI patient and three continuous treatment patients had mutations, mostly to lamivudine. When resistance mutations were tested in 126 patients who underwent at least two STI cycles, only seven had mutations and almost all were to lamivudine (M184V), with four patients also having NNRTI (Y181C, K103N) or PI (M46I, M36I) mutations. Compared with patients who received ritonavir-boosted saquinavir, the relative risk of resistance was 1.90 for NNRTI (P = 0.34) and 14.96 for triple NRTI regimens (P = 0.01).
Papasavvas et al. [27] reported more VL failure in the continuous arm (four out of 21) compared with the fixed interruption arm (one out of 21). In this cohort, 60% were on NNRTI regimens; all the failures were NNRTI treated. Twelve patients had no VL failure, but resistance mutations were detected during the long interruption: three out of 21 in the continuous arm and nine out of 21 in the fixed interruption arm. These were most likely archived mutations as the patients were drug experienced. Eleven out of 12 patients resumed the same therapy and achieved viral suppression.
ISS-PART saw a similar rate of virological failure in the STI compared with the continuous arms, but 40 out of 136 (29.4%) STI subjects developed mutations, corresponding to a 32% (95% confidence interval 23.5-40.0) cumulative risk over 24 months. In the STI arm, baseline residual replication greater than 2.5 copies/ml and plasma mutations during STI correlated with the risk of virological failure. Most patients already had archived mutations in proviral DNA at baseline, and this strongly predicted resistance mutation selection after STI (P = 0.001). The use of unboosted PI also predicted mutation selection (P = 0.040). These results should be interpreted with caution as resistance data on the continuous arm are lacking [28].
In Trivacan, at the 12-month interim analysis, 5 and 11% of continuous and CD4 cell count-guided arms had resistance mutations to at least one drug (P = 0.13). The trial is ongoing and there is no further information at this time [7].
Other scheduled treatment interruption risks
Acute retroviral syndrome can occur with high VL rebound during STI. In Staccato, acute retroviral syndrome was seen in 5.9%, confirmed with VL greater than 250 000 copies/ml in 2.3% [5]. The symptoms mimic acute HIV infection. Aseptic meningitis can occur [41,42]. Treatment is to resume HAART. Thrombocytopenia (without bleeding) can also occur during STI regardless of a previous history of thrombocytopenia [17,43]. Resuming HAART raises the platelet count.
The risk of transmission during VL rebound is a major public health concern [44,45]. Genital HIV concentrations increase in a stepwise fashion with declining CD4 cell counts [46]. Unprotected sex is not uncommon; it was reported in 12% of almost 5000 HIV-infected Swiss individuals surveyed [45]. A case of HIV transmission during treatment interruption has been reported in a discordant couple who stopped practising safe sex after the infected partner became VL undetectable on HAART, and continued to have unsafe sex during an STI cycle [44]. Therefore, an individual undergoing HAART interruption should be considered at a high risk of transmitting HIV during unprotected sex. It is the responsibility of the physician to enforce the clear message of safe sex during treatment interruption.
Table 2. Expectation & Reality in SMART

References
1. Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191-201.
[Context Link]
2. Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, et al. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 2002; 346:811-820.
[Context Link]
3. Mussini C, Pinti M, Bugarini R, Borghi V, Nasi M, Nemes E, et al. Effect of treatment interruption monitored by CD4 cell count on mitochondrial DNA content in HIV-infected patients: a prospective study. AIDS 2005; 19:1627-1633.
[Context Link]
4. Ananworanich J, Siangphoe U, Hill A, Cardiello P, Apateerapong W, Hirschel B, et al. Highly active antiretroviral therapy (HAART) retreatment in patients on CD4-guided therapy achieved similar virologic suppression compared with patients on continuous HAART: the HIV Netherlands Australia Thailand Research Collaboration 001.4 study. J Acquir Immune Defic Syndr 2005; 39:523-529.
[Context Link]
5. Ananworanich J, Gayet-Ageron A, Le Braz M, Prasithsirikul W, Chetchotisakd P, Kiertiburanakul S, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 2006; 368:459-465.
[Context Link]
6. Marchou B, Tangre P, Charreau I, Izopet J, Girard PM, May T, et al. Structured treatment interruptions in HIV-infected patients with high CD4 cell counts and virologic suppression: results of a prospective, randomized, open-label trial (Window-ANRS 106). In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, 5-8 February 2006 [Abstract 104].
[Context Link]
7. Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet 2006; 367:1981-1989.
[Context Link]
8. Dybul M, Nies-Kraske E, Dewar R, Maldarelli F, Hallahan CW, Daucher M, et al. A proof-of-concept study of short-cycle intermittent antiretroviral therapy with a once-daily regimen of didanosine, lamivudine, and efavirenz for the treatment of chronic HIV infection. J Infect Dis 2004; 189:1974-1982.
[Context Link]
9. Fagard C, Oxenius A, Gunthard H, Garcia F, Le Braz M, Mestre G, et al. A prospective trial of structured treatment interruptions in human immunodeficiency virus infection. Arch Intern Med 2003; 163:1220-1226.
[Context Link]
10. Delfraissy J. Symposium on structured treatment interruptions. In: XVth International AIDS Conference. Bangkok, Thailand, 12-16 July 2004 [Abstract TuSy188].
[Context Link]
11. Maggiolo F, Ripamonti D, Gregis G, Quinzan G, Callegaro A, Suter F. Effect of prolonged discontinuation of successful antiretroviral therapy on CD4 T cells: a controlled, prospective trial. AIDS 2004; 18:439-446.
[Context Link]
12. Ruiz L, Martinez-Picado J, Romeu J, Paredes R, Zayat MK, Marfil S, et al. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS 2000; 14:397-403.
[Context Link]
13. Garcia F, Plana M, Ortiz GM, Bonhoeffer S, Soriano A, Vidal C, et al. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS 2001; 15:F29-F40.
[Context Link]
14. Pai NP, Tulsky JP, Lawrence J, Colford JM Jr, Reingold AL. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database Syst Rev 2005; CD005482.
[Context Link]
15. El-Sadr W, Neaton J, for the SMART Study Investigators. Episodic CD4-guided use of antiretroviral therapy is inferior to continuous therapy: results of the SMART study. In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, 5-8 February 2006 [Abstract 106LB].
[Context Link]
16. Fagard C, Bandelier CY, Ananworanich J, Le Braz M, Gunthard H, Perneger T, et al. Biphasic decline of CD4 cell count during scheduled treatment interruptions. AIDS 2005; 19:439-441.
[Context Link]
17. Skiest D, Havlir D, Coombs R, Adams E, Cain P, Peterson T, et al. Predictors of HIV disease progression in patients who stop ART with CD4 cell counts > 350 cells/mm 3. In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, 5-8 February 2006 [Abstract 101].
[Context Link]
18. Vogler MA, Teppler H, Gelman R, Valentine F, Lederman MM, Pomerantz RJ, et al. Daily low-dose subcutaneous interleukin-2 added to single- or dual-nucleoside therapy in HIV infection does not protect against CD4+ T-cell decline or improve other indices of immune function: results of a randomized controlled clinical trial (ACTG 248). J Acquir Immune Defic Syndr 2004; 36:576-587.
[Context Link]
19. Achenbach CJ, Till M, Palella FJ, Knoll MD, Terp SM, Kalnins AU, Murphy RL. Extended antiretroviral treatment interruption in HIV-infected patients with long-term suppression of plasma HIV RNA. HIV Med 2005; 6:7-12.
[Context Link]
20. Foli A, Maserati R, Barasolo G, Castelli F, Tomasoni L, Migliorino, et al. Strategies to decrease viral load rebound, and prevent loss of CD4 and onset of resistance during structured treatment interruptions. Antivir Ther 2004; 9:123-132.
[Context Link]
21. Garcia F, Plana M, Arnedo M, Ortiz GM, Miro JM, Lopalco L, et al. A cytostatic drug improves control of HIV-1 replication during structured treatment interruptions: a randomized study. AIDS 2003; 17:43-51.
[Context Link]
22. Ruiz L, Romeu J, Martinez-Picado J, Bellido R, Llibre J, Domingo P, Tambussi G, Clotet B, and on behalf of the Tibet Study Group. Selection of Drug-resistance Mutations in Chronic HIV-infected Patients during Therapy Interruptions Guided by CD4+ T-cell Counts and Viral Load Levels: The Tibet Study. 12th Conference on Retroviruses and Opportunistic Infections, Boston, Feburary 22-25, 2004 [Abstract 679].
[Context Link]
23. Henry K, Katzenstein D, Cherng DW, Valdez H, Powderly W, Vargas MB, et al, A5102 Study Team of the AIDS Clinical Trials Group. A pilot study evaluating time to CD4 T-cell count < 350 cells/mm3 after treatment interruption following antiretroviral therapy ± interleukin 2: results of ACTG A5102. J Acquir Immune Defic Syndr 2006; 42:140-148.
[Context Link]
24. Ananworanich J, Nuesch R, Le Braz M, Chetchotisakd P, Vibhagool A, Wicharuk S, et al. Failures of 1 week on, 1 week off antiretroviral therapies in a randomized trial. AIDS 2003; 17:F33-F37.
[Context Link]
25. Cohen C, Colson A, Morris A, Alberts L, Sheble-Hall A, Hussey S, Schulte M. The five days on, two days off (FOTO) study: 48 week results: viral suppression can be maintained when antiretrovirals are taken for only 5 consecutive days each week. 3rd IAS Conference on HIV Pathogenesis and Treatment. Rio de Janeiro, Brazil [WePe12.4C10].
[Context Link]
26. Dybul M, Nies-Kraske E, Daucher M, Hertogs K, Hallahan CW, Csako G, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis 2003; 188:388-396.
[Context Link]
27. Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, et al. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med 2004; 1:e64.
[Context Link]
28. Palmisano L, Giuliano M, Bucciardini R, Andreotti M, Fragola V, Galluzzo C, et al. Final results of a randomized controlled trial of structured treatment interruption vs continuous HAART in chronic HIV-infected subjects with persistent suppression of viral replication. In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, 5-8 February 2006 [Abstract 103].
[Context Link]
29. The DART (Development of Anti-Retroviral Therapy in Africa) trial. DART trial moves patients from interrupted to continuous antiretroviral therapy (ART): 14 March 2006. Press release, available at: http://www.ctu.mrc.ac.uk/dart . Accessed: 23 May 2006.
[Context Link]
30. Muro E, Droste JA, Hofstede HT, Bosch M, Dolmans W, Burger DM. Nevirapine plasma concentrations are still detectable after more than 2 weeks in the majority of women receiving single-dose nevirapine: implications for intervention studies. J Acquir Immune Defic Syndr 2005; 39:419-421.
[Context Link]
31. Ribaudo HJ, Haas DW, Tierney C, Kim RB, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis 2006; 42:401-407.
[Context Link]
32. Jourdain G, Ngo-Giang-Huong N, Le Coeur S, Bowonwatanuwong C, Kantipong P, Leechanachai P, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med 2004; 351:229-240.
[Context Link]
33. Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med 2004; 351:217-228.
[Context Link]
34. Metzner KJ, Bonhoeffer S, Fischer M, Karanicolas R, Allers K, Joos B, et al. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. J Infect Dis 2003; 188:1433-1443.
[Context Link]
35. Martinez-Picado J, Morales-Lopetegi K, Wrin T, Prado JG, Frost SDW, Petropoulos CJ, et al. Selection of drug-resistant HIV-1 mutants in response to repeated structured treatment interruptions. AIDS 2002; 16:895-899.
[Context Link]
36. Yerly S, Fagard C, Gunthard HF, Hirschel B, Perrin L. Drug resistance mutations during structured treatment interruptions. Antivir Ther 2003; 8:411-415.
[Context Link]
37. Nuesch R, Ananworanich J, Sirivichayakul S, Ubolyam S, Siangphoe U, Hill A, et al. Development of HIV with drug resistance after CD4 cell count-guided structured treatment interruptions in patients treated with highly active antiretroviral therapy after dual-nucleoside analogue treatment. Clin Infect Dis 2005; 40:728-734.
[Context Link]
38. Cardiello PG, Hassink E, Ananworanich J, Srasuebkul P, Samor T, Mahanontharit A, et al. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin Infect Dis 2005; 40:594-600.
39. Hoen B, Fournier I, Lacabaratz C, Burgard M, Charreau I, Chaix ML, et al. Structured treatment interruptions in primary HIV-1 infection: the ANRS 100 PRIMSTOP trial. J Acquir Immune Defic Syndr 2005; 40:307-316.
40. Ananworanich J, Hirschel B, Furrer H, Ubolyam S, Gayet-Ageron A, Yerly S, et al. CD4-guided scheduled treatment interruptions: low incidence of resistance mutations in the Staccato trial. In: 13th Conference on Retroviruses and Opportunistic Infections. Denver, 5-8 February 2006 [Abstract 622B].
[Context Link]
41. Worthington MG, Ross JJ. Aseptic meningitis and acute HIV syndrome after interruption of antiretroviral therapy: implications for structured treatment interruptions. AIDS 2003; 17:2145-2146.
[Context Link]
42. Price RW, Paxinos EE, Grant RM, Drews B, Nilsson A, Hoh R, et al. Cerebrospinal fluid response to structured treatment interruption after virological failure. AIDS 2001; 15:1251-1259.
[Context Link]
43. Ananworanich J, Phanuphak N, Nuesch R, Apateerapong W, Rojnuckarin P, Ubolyam S, et al. Recurring thrombocytopenia associated with structured treatment interruption in patients with human immunodeficiency virus infection. Clin Infect Dis 2003; 37:723-725.
[Context Link]
44. Teicher E, Casagrande T, Vittecoq D. Enhanced risk of HIV sexual transmission during structured treatment interruption. Sex Transm Infect 2003; 79:74.
[Context Link]
45. Wolf K, Young J, Rickenbach M, Vernazza P, Flepp M, Furrer H, et al. Prevalence of unsafe sexual behavior among HIV-infected individuals: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2003; 33:494-499.
[Context Link]
46. McClelland RS, Baeten JM, Richardson BA, Lavreys L, Emery S, Mandaliya K, et al. A comparison of genital HIV-1 shedding and sexual risk behavior among Kenyan women based on eligibility for initiation of HAART according to WHO guidelines. J Acquir Immune Defic Syndr 2006; 41:611-615.
[Context Link]
|
|
| |
| |
|
|
|