| |
Effects of Continuing or Stopping Alendronate After 5 Years of Treatment
|
| |
| |
The Fracture Intervention Trial Long-term Extension (FLEX): A Randomized Trial
Dennis M. Black, PhD; Ann V. Schwartz, PhD; Kristine E. Ensrud, MD, MPH; Jane A. Cauley, DrPH; Silvina Levis, MD; Sara A. Quandt, PhD; Suzanne Satterfield, MD; Robert B. Wallace, MD; Douglas C. Bauer, MD, MPH; Lisa Palermo, MA; Lois E. Wehren, MD; Antonio Lombardi, MD; Arthur C. Santora, MD; Steven R. Cummings, MD; for the FLEX Research Group
JAMA. Dec 27, 2006;296:2927-2938.
AUTHOR CONCLUSION
We conclude that continuation of alendronate (either 5 or 10 mg/d) for 10 years maintains bone mass and reduces bone remodeling compared with discontinuation after 5 years. The results confirm the safety of alendronate for up to 10 years including no increased fracture risk with long-term alendronate use. However, even among those who discontinued therapy after 5 years, BMD remained at or above baseline values 10 years earlier and bone turnover was still somewhat reduced. Discontinuation did not increase the risk of nonvertebral fractures or x-ray-detected vertebral fractures over the next 5 years, but the risk of clinically diagnosed vertebral fractures was significantly increased among those who discontinued. These results suggest that for many women, discontinuation of alendronate after 5 years for up to 5 more years does not significantly increase fracture risk, but women at high risk of clinical vertebral fractures, such as those with vertebral fracture or very low BMD, may benefit by continuing beyond 5 years.
ABSTRACT
Context The optimal duration of treatment of women with postmenopausal osteoporosis is uncertain.
Objective To compare the effects of discontinuing alendronate treatment after 5 years vs continuing for 10 years.
Design and Setting Randomized, double-blind trial conducted at 10 US clinical centers that participated in the Fracture Intervention Trial (FIT).
Participants One thousand ninety-nine postmenopausal women who had been randomized to alendronate in FIT, with a mean of 5 years of prior alendronate treatment.
Intervention Randomization to alendronate, 5 mg/d (n = 329) or 10 mg/d (n = 333), or placebo (n = 437) for 5 years (1998-2003).
Main Outcome Measures The primary outcome measure was total hip bone mineral density (BMD); secondary measures were BMD at other sites and biochemical markers of bone remodeling. An exploratory outcome measure was fracture incidence.
Results
Compared with continuing alendronate, switching to placebo for 5 years resulted in declines in BMD at the total hip (-2.4%; 95% confidence interval [CI], -2.9% to -1.8%; P<.001) and spine (-3.7%; 95% CI, -4.5% to -3.0%; P<.001), but mean levels remained at or above pretreatment levels 10 years earlier.
Similarly, those discontinuing alendronate had increased serum markers of bone turnover compared with continuing alendronate: 55.6% (P<.001) for C-telopeptide of type 1 collagen, 59.5% (P < .001) for serum N = propeptide of type 1 collagen, and 28.1% (P<.001) for bone-specific alkaline phosphatase, but after 5 years without therapy, bone marker levels remained somewhat below pretreatment levels 10 years earlier.
After 5 years, the cumulative risk of nonvertebral fractures (RR, 1.00; 95% CI, 0.76-1.32) was not significantly different between those continuing (19%) and discontinuing (18.9%) alendronate. Among those who continued, there was a significantly lower risk of clinically recognized vertebral fractures (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24-0.85) but no significant reduction in morphometric vertebral fractures (11.3% for placebo and 9.8% for alendronate; RR, 0.86; 95% CI, 0.60-1.22). A small sample of 18 transilial bone biopsies did not show any qualitative abnormalities, with bone turnover (double labeling) seen in all specimens.
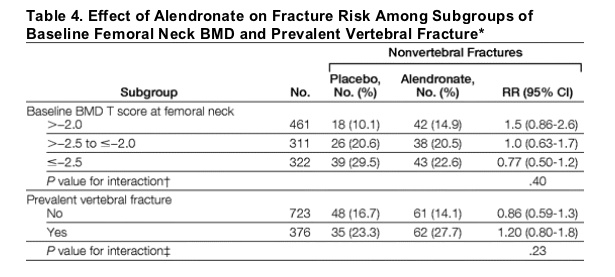
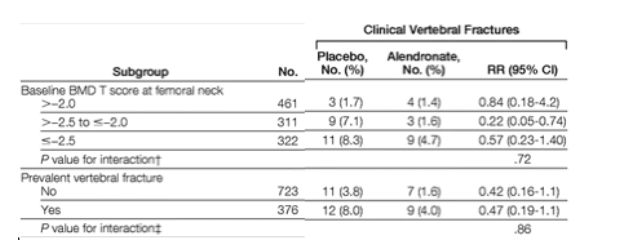
"....The post hoc subgroup fracture analysis did not show significant trends with lower BMD or prevalent vertebral fractures at FLEX baseline for either nonvertebral or clinical vertebral fractures (Table 4). However, the incidence of both types of fractures in the placebo group increased with lower baseline BMD or prevalent fracture...."see tables below
Conclusions Women who discontinued alendronate after 5 years showed a moderate decline in BMD and a gradual rise in biochemical markers but no higher fracture risk other than for clinical vertebral fractures compared with those who continued alendronate. These results suggest that for many women, discontinuation of alendronate for up to 5 years does not appear to significantly increase fracture risk. However, women at very high risk of clinical vertebral fractures may benefit by continuing beyond 5 years.
INTRODUCTION
Osteoporosis is common among postmenopausal women, and the disease process is characterized by increased bone turnover, progressive loss of bone mass, microarchitectural deterioration, and increased fracture risk. Bisphosphonates, which are antiresorptive drugs, are the most commonly used pharmacologic treatments for postmenopausal osteoporosis. Alendronate, a potent bisphosphonate, decreases bone turnover, increases bone mineral density (BMD), and decreases vertebral, nonspine, and hip fracture risk in women with osteoporosis.1-4
Treatment for osteoporosis often continues indefinitely, but few studies have examined the effects of using bisphosphonates longer than 5 years or the effects of stopping treatment after 5 years. A small number of participants in a phase 3 study of alendronate were followed up for as long as 10 years of continued treatment.5 Among women who continued alendronate, spinal BMD increased throughout the entire treatment period, with maintenance of increases in hip BMD. Women who discontinued treatment experienced small declines in hip BMD but maintained lumbar spine BMD. Similar BMD findings were reported from the 7-year follow-up of a small subset of participants in a study of risedronate.6 Few fracture risk data are available for long-term bisphosphonate treatment, although 1 study supported the long-term safety of alendronate by showing no increased fracture risk over 10 years.5
Pharmacokinetic studies show that bisphosphonates remain in bone matrix for many years, and the incorporated bisphosphonate remains inactive until it is gradually released as bone containing it is resorbed. The terminal half-life of alendronate is similar to that of bone mineral, approximately 10.5 years.7 Thus, some of the skeletal effects of alendronate and other bisphosphonates may last for years after treatment stops. These findings suggest that stopping treatment after 4 to 5 years might result in residual clinical efficacy, but the magnitude and duration of this effect remains uncertain. Because the effects of long-term use and discontinuation have been studied only in small numbers of participants who were not randomly assigned to continuation or discontinuation, available data are inadequate to determine optimal treatment duration.
The Fracture Intervention Trial (FIT), a randomized, blinded, placebo-controlled trial, examined the effect of daily alendronate on BMD and fracture risk in postmenopausal women with low BMD. Average follow-up during treatment was 3.8 years, with optional open-label treatment continuation after trial completion. In this article, we report data from the FIT Long-term Extension (FLEX), designed to evaluate in a randomized trial the effects on BMD of either continuation of alendronate, 5 or 10 mg/d for a total of 10 years, or discontinuation after approximately 5 years.
COMMENT
Among women previously treated for 5 years with alendronate, those who were randomly assigned to continue alendronate for 5 additional years maintained higher BMD at the hip and spine than those randomized to placebo. In those continuing alendronate, biomarkers of bone remodeling remained reduced, while those who stopped had a gradual increase in bone remodeling that, however, remained somewhat below pretreatment levels 10 years earlier.
The FLEX results are generally consistent with findings from a smaller long-term study of alendronate reported by Bone et al.5 In both studies, the difference in BMD between continuous alendronate for 10 years and treatment with alendronate for 5 years followed by placebo for 5 years was about 4% for spine BMD and about 3% to 4% at the hip. In addition, the 10-year increase in spine BMD for 10 mg/d of alendronate was about 14% in both studies. However, the within-group change in total hip and trochanter BMD was greater in the previous study than in FLEX; this may be due to the older age of the FLEX participants (about 6 years difference) and the fact that they were treated with only 5 mg/d for the first 2 years of FIT. Both studies also showed a rise in bone markers after discontinuation of alendronate. Although Bone et al5 found a difference between 10 years of 10 mg/d of alendronate compared with 10 years of 5 mg/d, in FLEX, women had similar BMD and marker responses during continued treatment with either 5 or 10 mg/d.
The changes in BMD and bone markers after discontinuation might provide some insight into the residual effect of alendronate following 5 years of use. While BMD at the hip decreased following discontinuation, the decrease was relatively small (2%-3% over 5 years). Thus, the cumulative effect of 5 years of alendronate followed by 5 years of placebo (1%-3% gain) was positive compared with the 5% to 10% loss expected from observational studies of untreated women of similar age.21 The changes in bone markers also suggest some residual effect after 5 years of treatment, although by the end of FLEX, bone marker levels were close to pretreatment (FIT baseline) values. Additionally, the FLEX placebo group was taking calcium and vitamin D, which can decrease bone marker levels by 10% to 30%.5, 22 However, because of the unknown effects of long-term storage of serum and the storage of FIT samples for a period at -20C, it is difficult to make an exact comparison to marker levels prior to the start of FLEX. The duration and extent of residual effects for bisphosphonates depends on cumulative dose and duration of treatment. Therefore, our results should not be generalized to shorter treatment periods or longer periods without treatment. Despite these limitations, the BMD and bone marker changes suggest some residual effect from 5 years of alendronate treatment that is evident for at least 5 years after discontinuation.21
The decline in BMD following discontinuation of alendronate was much lower than that seen after discontinuation of estrogen,23 raloxifene,24 or intermittent parathyroid hormone.25 Discontinuation of these agents is associated with immediate and substantial decreases in BMD. Gains in BMD during treatment appear to be better maintained after discontinuation of bisphosphonates, including alendronate,5, 23, 26 risedronate,27 pamidronate,28 and etidronate.29 Similarly, bone turnover markers increased only gradually after discontinuation of alendronate compared with sharp and immediate gains seen following estrogen, raloxifene, and parathyroid hormone discontinuation. Bisphosphonates bind strongly to hydroxyapatite. When bone containing bisphosphonate is resorbed, some of the bisphosphonate released recirculates locally and systemically and binds again to bone surfaces. Thus, when long-term treatment with bisphosphonates stops, the residual and recirculating bisphosphonate continues to inhibit bone resorption, although to a lesser extent than continued treatment. The retention of bisphosphonates in bone may account for the slow bone loss after bisphosphonates are stopped.5, 23, 27-29
We saw no difference in the rates of nonvertebral fractures. Although this finding must be tempered by the wide CIs (which cannot exclude a small increase or decrease in risk), it could be explained by the fact that changes in BMD and bone turnover after alendronate discontinuation for 5 years were gradual and may not have been sufficient to cause an increase in nonvertebral fracture risk compared with the alendronate group. Furthermore, effects on nonvertebral fracture risk have generally been demonstrated only among patients with pretreatment osteoporosis, as assessed by BMD, or preexisting vertebral fractures.2-3,22, 30-31 Many FLEX participants did not have osteoporosis, either because they entered the FIT trial without osteoporosis or because they experienced gains in BMD during FIT, and this would further reduce the power to detect a difference between groups, if one exists.
The incidence of clinical vertebral fractures was low (5.3% over 5 years in placebo) but was reduced to 2.4% in those who continued taking alendronate, an RR reduction of 55% and an absolute risk reduction of 2.9%. Among women with existing vertebral fractures or very low BMD who are at much higher risk of future vertebral fractures,32 the absolute benefit for clinical vertebral fractures is higher.
Radiographically defined vertebral fractures were more common in the placebo group (about 11%), but the 14% reduction among those continuing alendronate was not statistically significant. However, the CIs for reductions in clinical vs morphometeric vertebral fractures are wide and overlapping and, thus, these results may not be inconsistent.
Decreased bone turnover has been associated with decreased fracture risk independent of BMD effects,33-34 and among women treated for osteoporosis, greater reductions in turnover are associated with fewer fractures.35-36 Potential mechanisms include allowing resorption pits to fill with bone and reduction in the depth and size of new resorption sites. Conversely, there has been recent controversy about the effect of long-term reduction of bone turnover on bone strength. It has been suggested that long-term reduced bone turnover might decrease bone strength by allowing microcracks to accumulate,37 although others have suggested that increased microcrack density might decrease fracture risk.38-39 The net effect of long-term decrease in bone turnover on bone strength would be best tested in trials comparing long-term treatment with bisphosphonates and placebo. However, the absence of an increased risk of nonvertebral fracture with 10 years of treatment compared with stopping after 5 years, as well as the decreased risk of clinical vertebral fracture in those continuing alendronate, suggests that continuing treatment for 10 years does not have adverse effects on bone strength.
While osteonecrosis of the jaw has been recently associated with bisphosphonate treatment, the vast majority of cases occur in cancer patients receiving frequent intravenous bisphosphonates, and the risk among osteoporosis patients using oral bisphosphonates is very low.40 That we saw no cases of osteonecrosis of the jaw supports the view that even long-term use of oral bisphosphonates carries little risk of osteonecrosis of the jaw.
This trial has several limitations. The effect of alendronate on fracture risk was an exploratory aim and the trial had limited power to detect modest differences in fracture rates, reflected in the wide CIs for fracture outcomes. The FIT participants received 5 mg/d of alendronate for the first 2 years, and results with 10 mg/d for 10 years may have differed. Finally, the average age of participants at baseline was 73 years, so results might not apply to younger women, men, or the very elderly.
We did not see any subgroups of patients in whom the relative fracture benefits of continuation were greater, although our power for subgroup analyses is limited. However, clinical vertebral fractures were more common, and the absolute benefit for them was larger in women with existing vertebral fractures or those with very low FLEX baseline BMD. Other ongoing analyses are examining if other factors can identify patients in whom continuation has greater benefits.
METHODS
Participants
Details of the design and outcomes of FIT, as well as interim results from FLEX, have previously been reported.2-3,8-9 For the FIT study, postmenopausal women aged 55 to 81 years with low femoral neck BMD (<0.68 g/cm2) were eligible to participate. Of 6459 enrolled participants, 2027 women with 1 or more preexisting vertebral deformities were enrolled in the vertebral fracture arm and 4432 women with no existing vertebral deformity were enrolled in the clinical fracture arm. In each arm, women were randomized to alendronate, 5 mg/d for 2 years and 10 mg/d thereafter (n = 3236), or placebo (n = 3223). Average follow-up in the FIT vertebral fracture arm was 2.9 years and in the clinical fracture arm was 4.2 years. One year of alendronate, 10 mg/d, was offered at no cost to all participants at the end of FIT. Thereafter, women were encouraged to consult their personal physicians regarding continued treatment.
Eligibility in FLEX was limited to women assigned to receive alendronate during FIT who completed at least 3 years of treatment during the trial and subsequent open-label period. Women whose total hip BMD at FLEX baseline was less than 0.515 g/cm2 (T score <-3.5)10 or whose total hip BMD was lower than at FIT baseline were ineligible. Because fracture risk and bone density differ by race, women were asked to report their race/ethnicity during the baseline visit. This self-reported race/ethnicity was recorded by the interviewer as 1 of the following categories: white, black or African American, Hispanic or Latina, Asian or Pacific Islander, or other (specified). Ten of the 11 original clinical centers in FIT participated in FLEX. A total of 1099 women were enrolled in FLEX (Figure 1). All women provided written informed consent, and the protocol was approved by the appropriate institutional review boards.
Treatment
At FLEX baseline, participants were randomly allocated (using a permuted-block design, stratified by study stratum and center) to receive alendronate, 10 mg/d (30%), alendronate, 5 mg/d (30%), or placebo (40%) for 5 years. Each participant was also offered a daily supplement containing 500 mg of calcium and 250 U of vitamin D. Two randomization strata were defined: the higher-risk stratum included women with 1 or more morphometric vertebral deformities at the end of FIT or with a clinical fracture during FIT; all other women were randomized to the low-risk stratum.
Participants and all study staff and investigators, except a senior statistician, remained blinded to treatment allocation and BMD follow-up values throughout the study. The senior statistician created unblinded reports that were reviewed periodically by a data monitoring committee. The 3-year interim analysis9 was performed without unblinding of investigators to individual assignments.9
Follow-up
Annual examinations were conducted at clinical centers. Every 3 months, women were contacted by telephone to encourage adherence, identify adverse experiences (including fractures), and update concurrent medication use. Adherence was assessed in the clinic by self-report and pill count.
BMD Measurement
At FLEX baseline, BMD was measured at the posteroanterior lumbar spine, hip (femoral neck, trochanter, total), and total body using dual-energy x-ray absorptiometry. Forearm BMD was measured in 40% of participants. Measurements were made with the same Hologic QDR 2000 densitometers used for FIT (Hologic Inc, Bedford, Mass). Hip BMD was measured annually; spine, total body, and forearm BMD were measured at the 36- and 60-month visits. Total hip BMD was the primary end point, while BMD measurements at other sites were secondary end points.
Dual-energy x-ray absorptiometry quality control was the same as that used in FIT, including technician training and certification, daily scanning of local spine and hip phantoms, baseline scanning of a study-wide phantom at each center, and review of a random sample of scans. In FLEX, phantom-based reproducibility of spine BMD was 0.4% to 0.5%, and for similar machines in other studies, in vivo reproducibility has been reported as about 1%.11-13 If a participant experienced excessive bone loss at the total hip (loss >8% over 1 year, 10% over 2 years, etc) or 3 or more new fractures, the investigator was notified without disclosing treatment assignment. Risks and benefits associated with study continuation were discussed with the participant. Discontinuation from study drug was required if any total hip BMD measurement was more than 5% below the FIT baseline value.
Stature was assessed using a wall-mounted stadiometer.8
Biochemical Markers of Bone Turnover
At the end of FLEX, analyses of biochemical markers (secondary end points) were performed in 1 batch using stored serum from FIT and FLEX. Of the 1099 participants in FLEX, a sample of 236 participants were chosen who had a complete set of samples (at FIT baseline and at end of FIT study, either 36 months or 48 months; at FLEX baseline and at 36 and 60 months) and were adherent throughout the study (defined as having taken >75% of assigned study medication). Specimens were obtained in a nonfasting state and stored at -70C during FLEX. During FIT, samples were stored at -70C with the exception of 2 years at -20C. All assays were performed at a central laboratory (Synarc, Lyon, France). Serum C-terminal telopeptide of type 1 collagen, a marker of bone resorption, and N-propeptide of type 1 collagen, a marker of bone formation, were measured with 2-site immunoassays on an automatic analyzer (Eleccys, Riagnostic, Mannheim, Germany). Bone-specific alkaline phosphatase was measured by the Ostase assay (Beckman, San Diego, Calif). Intra-assay and interassay coefficients of variability for serum N-propeptide of type 1 collagen and serum C-terminal telopeptide of type 1 collagen are approximately 4% and 6%, respectively.
During the study, 2 other bone markers were assayed at several time points, and interim results were previously reported.9 However, because of concerns about assay drift with assays performed over several years, these analyses are not included herein.
Fractures
Fracture incidence in the alendronate and placebo groups was an exploratory objective, including clinical and morphometric vertebral fractures, all nonvertebral fractures, forearm fractures, and hip fractures. Potential fractures were identified by self-report and confirmed by radiology or surgical reports. Pathological, skull, and excessive trauma fractures were excluded. Potential clinical spine fractures were identified by diagnosis of vertebral fracture by a participant's physician, usually following reported back pain. A copy of the spine radiograph used by the participant's physician to diagnose the fracture was compared with radiographs from FLEX baseline. Clinical spine fractures were confirmed if the semiquantitative grading of vertebral fractures14 had increased by at least 1 category from baseline.
Lateral spine radiographs were obtained at FLEX baseline and at 36 and 60 months for morphometric vertebral fracture ascertainment. Six points were placed on each vertebra to define anterior, posterior, and mid heights (Synarc Inc) and radiographs with suspected incident morphometric fractures were also given semiquantitative grades.14-15 A new fracture was defined as a decrease of more than 20% in any vertebral height (minimum, 4 mm) with a semiquantitative confirmation. Prevalent vertebral fracture at baseline was defined as either randomization to the FIT vertebral fracture arm or deformity on baseline FLEX radiograph derived from quantitative morphometry.16-17
Bone Biopsies
Transilial bone biopsies, 7.5 mm in diameter, were obtained at the conclusion of FLEX using standard techniques.18 Only 1 of the 10 sites chose to participate in the bone biopsy study, and a total of 29 samples were obtained; 18 were of sufficient quality for histomorphometry. To assess bone formation, double fluorochrome label was administered prior to biopsy. Two-dimensional histomorphometry was performed at Creighton University, Omaha, Neb, using standard techniques.19
Adverse Experiences
Adverse events were defined as occurrence of any untoward condition, including minor illnesses, during the course of the study. All information on adverse experiences was gathered at each patient contact, and specific categories were defined for analysis. These included 3 general categories: serious (fatal, life-threatening, or those requiring or prolonging hospitalization); those considered by clinical investigators as definitely, probably, or possibly related to study medication; and those resulting in study medication discontinuation. Upper gastrointestinal tract adverse events were also identified by specific symptoms and diagnoses.
Statistical Analyses
Modified intention-to-treat analysis, using all available data from all participants assigned to treatment who had at least 1 follow-up measure, regardless of study medication adherence, was used for analysis of BMD. If at least 1 postrandomization value was available, we carried forward values for later missing values. In addition, we performed sensitivity analyses without carrying forward BMD values or excluding women who discontinued study drug or used other bone-active medications. The analysis of biochemical markers was based on a subgroup of women with high adherence and is therefore de facto a per-protocol analysis. As prespecified, data from both alendronate dosage groups were pooled for primary analyses; secondary analyses for BMD and biochemical markers were performed without pooling. Any significant differences between doses are noted herein.
Baseline characteristics by FLEX treatment group were compared by 2 tests and analysis of variance. As prespecified in the data analysis plan, percentage change in BMD from FLEX baseline and log fraction of baseline values for bone turnover markers were analyzed using an analysis of variance model with factors for treatment assignment, study center, and fracture risk stratum. Treatment differences were estimated by differences in least-square means from the analysis of variance model and by calculating 95% confidence intervals (CIs). Clinical fractures were analyzed using the Kaplan-Meier estimator and the log-rank test to compare treatment groups. The effect of alendronate was evaluated using proportional hazards models for clinical fractures and logistic regression models for morphometric vertebral fractures, adjusted for clinic and stratum. Results are reported as relative risks (RRs).
Comparison of the proportion of participants with morphometric vertebral fractures was made using RRs and the Mantel-Haenszel 2 statistic. 2 or Fisher exact tests were used for between-group comparisons of adverse events. The mean percentage change in BMD from FIT baseline to the completion of FLEX was also analyzed. Fracture efficacy was analyzed within subgroups of FLEX baseline BMD T score and by FLEX baseline vertebral fracture.3, 20
With a sample size of 1099, 4% observed standard deviation BMD, and a 2-sided level of .05, the trial had 90% power to detect a difference of 0.9% change in total hip BMD between the combined alendronate vs placebo groups. Based on 20% incidence of fracture in placebo, the trial had 80% power to detect a risk reduction of 33% to 13.5%.
RESULTS
Study Participants and Adherence
A total of 1099 women, all of whom had previously received alendronate, were enrolled in FLEX and rerandomized to receive placebo (n = 437), alendronate, 5 mg/d (n = 329), or alendronate, 10 mg/d (n = 333) (Figure 1). The average age was 73 years, 61% had self-rated very good or excellent health, 34% had prevalent vertebral fractures, and 60% had a history of clinical fractures since menopause (Table 1). At FLEX baseline, total hip BMD averaged 0.73 g/cm2 (T score, -1.9); femoral neck BMD, 0.61 g/cm2 (T score, -2.2); and lumbar spine BMD, 0.90 g/cm2 (T score, -1.3). On average, participants had taken alendronate for 5 years during and after FIT and 78% were currently using alendronate. There were no significant differences between treatment groups. Screening and randomization occurred in 1998 and the last participant finished in October 2003.
Of the 1099 randomized participants, 914 women (87% of survivors) had measurement of total hip BMD (the primary study end point) at the 60-month visit and 759 (72% of survivors) were still taking study medication. This proportion did not vary by treatment assignment (Figure 1). In the placebo group, 83 of 102 who discontinued study drug took bone-active medications, including alendronate, at some point during the study.
Bone Mineral Density
Among women enrolled in FLEX, all of whom received alendronate during FIT, 5 additional years of alendronate (5- and 10-mg groups combined) maintained BMD at the total hip compared with placebo (mean difference, 2.36%; 95% CI, 1.81%-2.90%; P<.001) (Table 2). Women randomized to alendronate had a mean total hip BMD decline of 1.02% compared with 3.38% with placebo. The patterns of change in femoral neck and trochanteric BMD were similar, with mean differences of 1.94% (95% CI, 1.20%-2.68%) and 3.17% (95% CI, 2.49%-3.84%), respectively (P<.001 for both). Lumbar spine BMD increased by 5.26% in the alendronate group compared with 1.52% in the placebo group, a mean difference of 3.74% (95% CI, 3.03%-4.45%; P<.001). Similar statistically significant mean differences between the alendronate and placebo groups were observed for total body and forearm BMD (1.28%; 95% CI, 0.70%-1.86%; and 2.01%; 95% CI, 1.35%-2.68%; P<.001 for both). With the exception of total body BMD, only small differences were noted between the 5- and 10-mg alendronate groups. Change in BMD from FIT baseline through the completion of FLEX is shown in Figure 2. At each site, BMD gains after 10 years of alendronate were significantly greater than after 5 years of alendronate followed by 5 years of placebo. Total hip BMD increased 2.41% among women treated with alendronate in FIT and FLEX compared with a decrease of 0.16% among women treated with placebo in FLEX (mean difference, 2.57%; 95% CI, 1.78%-3.36%; P<.001). Both groups experienced net gains in BMD from FIT baseline at the femoral neck (4.75% with alendronate vs 2.50% with placebo) and trochanter (5.95% with alendronate vs 2.62% with placebo). Overall BMD gains were greatest at the lumbar spine: 14.80% for alendronate vs 10.99% for placebo (difference, 3.81%; 95% CI, 2.64%-4.97%; P<.001). Women randomized to alendronate gained 3.60% total body BMD compared with 2.48% gains in the placebo group (difference, 1.12%; 95% CI, 0.34%-1.91%; P = .005).
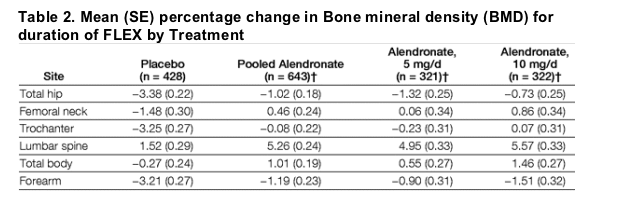
Figure 2. BMD Change in FLEX Participants
BMD indicates bone mineral density; FIT, Fracture Intervention Trial; FLEX, Fracture Intervention Trial Long-term Extension. Error bars indicate 95% confidence intervals. Data are shown for the period spanning the beginning of FIT through the completion of FLEX, a total of 10 years.
*Measured in clinical fracture arm only.
Sensitivity analyses limited to those who remained taking study medication and those who did not use bone-active medications, as well as analyses that did not carry data forward to replace missing values, yielded similar results (data not shown).
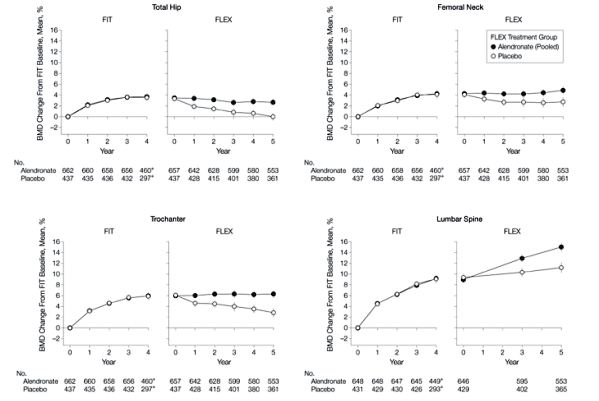
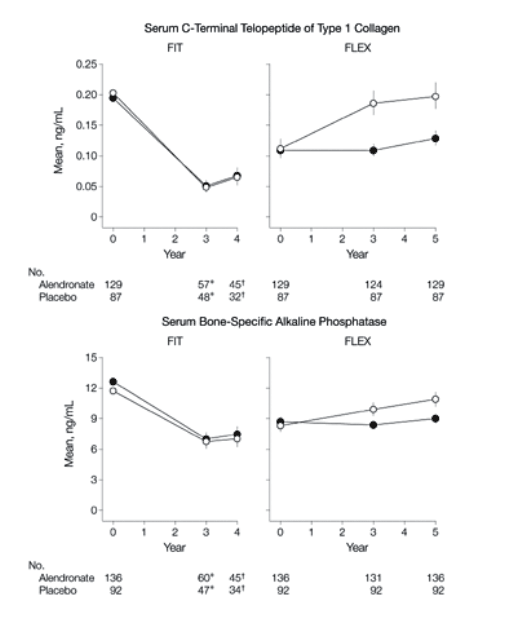
Biochemical Markers of Bone Turnover
Results for the 3 biochemical markers were similar (Figure 3). Those continuing alendronate remained at levels close to FLEX baseline, while those discontinuing alendronate experienced a gradual rise in markers over 5 years (difference compared with continued alendronate, 55.6% for serum C-terminal telopeptide of type 1 collagen, 59.5% for N-propeptide of type 1 collagen, and 28.1% for bone-specific alkaline phosphatase). When compared with pretreatment levels in FIT 10 years earlier, markers at the end of FLEX in those who discontinued were somewhat lower (-7% for serum C-terminal telopeptide of type 1 collagen and bone-specific alkaline phosphatase and -24% for N-propeptide of type 1 collagen compared with FIT baseline) (Figure 3). There was no significant difference between those taking 5 mg/d and 10 mg/d of alendronate for any markers.
Figure 3. Biochemical Markers of Bone Turnover in FLEX Participants
FIT indicates Fracture Intervention Trial. Error bars indicate 95% confidence intervals. Data are shown for the period spanning the beginning of FIT through the completion of FLEX
*Measured in vertebral fracture arm only.
Measured in clinical fracture arm only.
Fractures
No significant differences between treatment groups were observed for all clinical fractures or nonvertebral fractures (nonvertebral fractures, 19.0% with placebo vs 18.9% with alendronate; RR, 1.00; 95% CI, 0.76-1.32) (Table 3 and Figure 4). There was a statistically significantly lower risk of clinical vertebral fractures among those randomized to alendronate (5.3% with placebo vs 2.4% with alendronate; RR, 0.45; 95% CI, 0.24-0.85), although the small decrease in morphometric vertebral fractures among those taking alendronate was not significant (11.3% with placebo vs 9.8% with alendronate; RR, 0.86; 95% CI, 0.60-1.22).
The post hoc subgroup fracture analysis did not show significant trends with lower BMD or prevalent vertebral fractures at FLEX baseline for either nonvertebral or clinical vertebral fractures (Table 4). However, the incidence of both types of fractures in the placebo group increased with lower baseline BMD or prevalent fracture.
To compare nonvertebral fracture incidence in FIT and FLEX, we ran proportional hazards models among alendronate-treated participants with study and age as predictors and found that after adjustment for age, fracture incidence was similar in the 2 studies.
There was not a significant difference in height loss between the treatment groups.
Histomorphometry/Micro-Computed Tomography
Eighteen iliac crest biopsy specimens were suitable for 2-dimensional histomorphometry (9 placebo and 9 combined alendronate). No significant quantitative differences were seen, although numbers are small and CIs wide (Table 5). Dual labeling was present in all specimens.
Safety
No significant between-group differences were seen in serious adverse experiences, discontinuations due to adverse events, or rates of death, nor were differences seen in upper gastrointestinal tract or serious upper gastrointestinal tract adverse experiences (data not shown). No cases of osteonecrosis of the jaw were observed.
Author Affiliations: San Francisco Coordinating Center (Drs Black, Schwartz, Bauer, and Cummings and Ms Palermo), Departments of Epidemiology and Biostatistics (Drs Black and Schwartz and Ms Palermo) and Medicine (Dr Bauer), University of California, San Francisco, and California Pacific Medical Center Research Institute (Dr Cummings), San Francisco; Department of Medicine, VA Medical Center, Minneapolis, Minn (Dr Ensrud); Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pa (Dr Cauley); Department of Medicine, University of Miami, Miami, Fla (Dr Levis); School of Medicine, Wake Forest University, Winston-Salem, NC (Dr Quandt); Department of Preventive Medicine, University of Tennessee, Memphis (Dr Satterfield); College of Medicine, University of Iowa, Iowa City (Dr Wallace); and Merck Research Laboratories, Rahway, NJ (Drs Wehren, Lombardi, and Santora).
REFERENCES
1. Liberman UA, Weiss SR, Broll J, et al, Alendronate Phase III Osteoporosis Treatment Study Group. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437-1443. FREE FULL TEXT
2. Black DM, Cummings SR, Karpf DB, et al, Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541. FULL TEXT
| ISI | PUBMED
3. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077-2082. FREE FULL TEXT
4. Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE. Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int. 2005;16:468-474. FULL TEXT
| ISI | PUBMED
5. Bone HG, Hosking D, Devogelaer JP, et al. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350:1189-1199. FREE FULL TEXT
6. Mellstrom DD, Sorensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462-468. FULL TEXT
| ISI | PUBMED
7. Rodan G, Reszka A, Golub E, Rizzoli R. Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin. 2004;20:1291-1300. FULL TEXT
| ISI | PUBMED
8. Black DM, Reiss TF, Nevitt MC, Cauley J, Karpf D, Cummings SR. Design of the Fracture Intervention Trial. Osteoporos Int. 1993;3:S29-S39. PUBMED
9. Ensrud KE, Barrett-Connor EL, Schwartz A, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial Long-Term Extension. J Bone Miner Res. 2004;19:1259-1269. FULL TEXT
| ISI | PUBMED
10. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489. FULL TEXT
| ISI | PUBMED
11. Hans D, Duboeuf F, Schott AM, et al. Effects of a new positioner on the precision of hip bone mineral density measurements. J Bone Miner Res. 1997;12:1289-1294. FULL TEXT
| ISI | PUBMED
12. Devogelaer JP, Baudoux C, Nagant de Deuxchaisnes C. Reproducibility of BMD measurements on the Hologic QDR2000. Presented at: 3rd Bath Conference on Osteoporosis and Bone Mineral Measurements, June 23-26, 1992, Bath, England.
13. Franck H, Munz M, Scherrer M. Evaluation of dual-energy x-ray absorptiometry bone mineral measurement-comparison of a single-beam and fan-beam design: the effect of osteophytic calcification on spine bone mineral density. Calcif Tissue Int. 1995;56:192-195. FULL TEXT
| ISI | PUBMED
14. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137-1148. ISI | PUBMED
15. Black DM, Cummings SR, Stone K, Hudes E, Palermo L, Steiger P. A new approach to defining normal vertebral dimensions. J Bone Miner Res. 1991;6:883-892. ISI | PUBMED
16. Eastell R, Cedel SC, Wahner HW, Riggs BL, Melton LJ. Classification of vertebral fractures. J Bone Miner Res. 1991;6:207-215. ISI | PUBMED
17. Black DM, Palermo L, Nevitt MC, et al. Comparison of methods for defining prevalent vertebral deformities: the Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:890-902. ISI | PUBMED
18. Hodgson SF, Johnson KA, Muhs JM, Lufkin EG, McCarthy JT. Outpatient percutaneous biopsy of the iliac crest: methods, morbidity, and patient acceptance. Mayo Clin Proc. 1986;61:28-33. ISI | PUBMED
19. Recker RR. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, Fla: CRC Press; 1983.
20. Black DM, Thompson DE, Bauer DC, et al, FIT Research Group. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85:4118-4124. FREE FULL TEXT
21. Ensrud KE, Palermo L, Black DM, et al. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the Study of Osteoporotic Fractures. J Bone Miner Res. 1995;10:1778-1787. ISI | PUBMED
22. Harris ST, Watts NB, Genant HK, et al, Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344-1352. FREE FULL TEXT
23. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883. FREE FULL TEXT
24. Neele SJ, Evertz R, De Valk-De Roo G, Roos JC, Netelenbos JC. Effect of 1 year of discontinuation of raloxifene or estrogen therapy on bone mineral density after 5 years of treatment in healthy postmenopausal women. Bone. 2002;30:599-603. FULL TEXT
| ISI | PUBMED
25. Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555-565. FREE FULL TEXT
26. Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11:622-630. FULL TEXT
| PUBMED
27. Mortensen L, Charles P. Bioavailability of calcium supplements and the effect of vitamin D: comparisons between milk, calcium carbonate, and calcium carbonate plus vitamin D. Am J Clin Nutr. 1996;63:354-357. ABSTRACT
28. Landman JO, Hamdy NA, Pauwels EK, Papapoulos SE. Skeletal metabolism in patients with osteoporosis after discontinuation of long-term treatment with oral pamidronate. J Clin Endocrinol Metab. 1995;80:3465-3468. ABSTRACT
29. Miller PD, Watts NB, Licata AA, et al. Cyclical etidronate in the treatment of postmenopausal osteoporosis: efficacy and safety after seven years of treatment. Am J Med. 1997;103:468-476. FULL TEXT
| ISI | PUBMED
30. Neer M, Slovik DM, Daly M, Potts T Jr, Nussbaum SR. Treatment of postmenopausal osteoporosis with daily parathyroid hormone plus calcitriol. Osteoporos Int. 1993;3(suppl 1):204-205. ISI | PUBMED
31. Chesnut CH III, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241-1249. FULL TEXT
| ISI | PUBMED
32. Black DM, Arden NK, Palermo L, Pearson J, Cummings SR, Study of Osteoporotic Fractures Research Group. Prevalent vertebral deformities predict hip fractures and new deformities but not wrist fractures. J Bone Miner Res. 1999;14:821-828. FULL TEXT
| ISI | PUBMED
33. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996;11:1531-1538. ISI | PUBMED
34. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526-1536. FULL TEXT
| ISI | PUBMED
35. Bauer DC, Garnero P, Hochberg MC, et al. Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res. 2006;21:292-299. FULL TEXT
| ISI | PUBMED
36. Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res. 2003;18:1051-1056. FULL TEXT
| ISI | PUBMED
37. Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2-4. FULL TEXT
| ISI | PUBMED
38. Sobelman OS, Gibeling JC, Stover SM, et al. Do microcracks decrease or increase fatigue resistance in cortical bone? J Biomech. 2004;37:1295-1303. FULL TEXT
| ISI | PUBMED
39. Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185-191. FULL TEXT
| ISI | PUBMED
40. Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753-761. FREE FULL TEXT
|
|
| |
| |
|
|
|