| |
Cervical Shedding of HIV-1 RNA Among Women With Low Levels of Viremia While Receiving HAART
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 44(1) 1 January 2007 pp 38-42
Neely, Michael N MD*; Benning, Lorie PhD; Xu, Jiaao MD*; Strickler, Howard D MD; Greenblatt, Ruth M MD; Minkoff, Howard MD; Young, Mary MD; Bremer, James MD; Levine, Alexandra M MD**; Kovacs, Andrea MD
From the *Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA; Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY; Department of Medicine and Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, CA; Department of Obstetrics and Gynecology, Maimonides Medical Center, Brooklyn, NY; Department of Medicine, Georgetown University Medical Center, Washington, DC; Department of Immunology and Microbiology, Rush University Medical Center, Chicago, IL; **Department of Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA; and Department of Pediatrics and Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA.
"....In summary, we have demonstrated that 44 (15%) of a cohort of 290 women on HAART with plasma HIV-1 RNA levels <500 copies/mL were shedding cervical HIV-1 RNA at a single time point. The risk of cervical HIV-1 RNA shedding was independently increased by use of NNRTI-based HAART versus PI-based HAART. The common occurrence of cervical viral RNA shedding in this cohort has implications for counseling women about the possibility of HIV transmission to infants or sexual partners or the spread of resistant virus from the sanctuary of the genital tract, even when plasma viral replication is effectively suppressed. The finding of more frequent cervical viral RNA shedding among women treated with an NNRTI-based antiretroviral regimen deserves further prospective investigation...."
Abstract
Background: Among women with low or undetectable quantities of HIV-1 RNA in plasma, factors associated with genital HIV-1 RNA shedding, including choice of treatment regimen, are poorly characterized.
Methods: We measured HIV-1 RNA in cervical swab specimens obtained from participants in the Women's Interagency HIV Study who had concurrent plasma viral RNA levels <500 copies/mL, and we assessed factors associated with genital HIV shedding. The study was powered to determine the relative effects of antiretroviral protease inhibitors (PIs) versus nonnucleoside reverse transcriptase inhibitors (NNRTIs) on viral RNA shedding.
Results: Overall, 44 (15%) of 290 women had detectable HIV-1 RNA in cervical specimens. In the final multivariate model, shedding was independently associated with NNRTI (vs. PI) use (odds ratio [OR], 95% confidence interval [CI]: 2.24, 1.13 to 4.45) and illicit drug use (OR, 95% CI: 2.41, 0.96 to 5.69).
Conclusions: This is the largest study to define risks for genital HIV-1 RNA shedding in women with low/undetectable plasma virus. Shedding in this population was common, and NNRTI-based highly active antiretroviral therapy (HAART) (vs. PI-based HAART) was associated with genital HIV shedding. Further study is required to determine the impact of these findings on transmission of HIV from mother to child or to sexual partners.
Introduction
As we and others have demonstrated, HIV-1 viral replication in plasma and female genital secretions is not equivalent.1 In our previous study, 158 (64%) of 247 women had detectable viral RNA in cervical secretions at a single time and plasma HIV-1 RNA was the most important determinant of cervical HIV-1 shedding.1 Of the 83 women with low or undetectable plasma viral RNA (<500 copies/mL), however, 27 (33%) had detectable cervical viral RNA.
Although the risk of HIV transmission to sexual partners2 or to a woman's newborn infant3 is proportional to the plasma viral RNA level, vertical transmission from mother to fetus has been documented even with undetectable plasma virus.4 The risk, albeit low, of transmission in this setting may be related to the discordance between plasma and cervical viral replication. Therefore, it is critical in the current highly active antiretroviral therapy (HAART) era to understand factors that have an impact on cervical shedding of HIV-1 RNA in women with low or undetectable plasma HIV-1 RNA better and whether the frequency of cervical shedding differs by type of antiretroviral therapy (ART) and/or other treatment-related factors.
To identify factors associated with cervical HIV-1 RNA shedding in women with low and undetectable plasma HIV-1 RNA better, we investigated a larger cohort than in our previous study.1 In particular, because of differences between nonnucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor (PI) penetration into semen,5 this study was designed to test the hypothesis that among treated HIV-infected women with a plasma viral load of <500 copies/mL (and therefore presumed good adherence), the percentage with cervical HIV-1 RNA shedding would differ between those taking NNRTI-containing HAART versus PI-containing HAART.
DISCUSSION
This study demonstrated that in a large cohort of treated HIV-infected women with low or undetectable plasma viral RNA, 15% were nonetheless shedding viral RNA from the cervix and that NNRTI use (vs. PI use) correlated with cervical viral RNA shedding. We have previously shown that the presence of genital HIV-1 RNA correlates with shedding of culturable infectious HIV.1 Therefore, the current data suggest that in the HAART era, women with low or undetectable plasma virus may still be at risk, albeit low, for transmitting HIV to their sexual partners or infants; indeed, this has already been reported for vertical transmission.16 Our finding that genital viral RNA shedding was related to the choice of antiretroviral regimen implies that future assessments of the effects of novel antiretroviral agents or combinations of agents could include genital viral replication in addition to plasma replication, especially because divergence between plasma and genital HIV quasispecies has been demonstrated.17-19
The number of shedders is lower in this cohort than previously reported,1,20,21 most likely because all participants in our cohort were treated with HAART that effectively reduced plasma viral replication. Potential treatment-related confounders, such as adherence and length of therapy before the swab visit, were not significant predictors of cervical RNA shedding in the univariate or multivariate analysis.
Recent data suggest that the penetration of antiretrovirals into cervicovaginal secretions is drug specific, with nevirapine, zidovudine, lamivudine, and emtricitabine demonstrating greater penetration than efavirenz or PIs.22,23 Our study was not powered to detect differences in cervical HIV-1 RNA shedding related to specific drugs, and we did not measure concentrations of antiretrovirals in the genital tract; however, a trend toward increased shedding among women in this cohort treated with efavirenz (see Table 1) is consistent with the pharmacokinetic data. In contrast, our finding of decreased cervical HIV-1 viral RNA in women treated with PIs is unexpected, given the reportedly decreased PI penetration into female genital secretions,22,23 and indicates that studies are required to measure drug concentrations and HIV-1 RNA simultaneously in the female genital tract. This pharmacokinetic-pharmacodynamic relation is the subject of a planned future investigation.
Although there were significant differences between early and expansion recruits, none of these factors were independently associated with shedding in univariate or multivariate analysis; therefore, it is likely that period of enrollment was a surrogate for a combination of these exposures and/or unmeasured cofactors, such as archived antiretroviral resistance, because these women had been relatively heavily mono- or dual-ART exposed before receiving HAART. Although the WIHS cohort is followed prospectively, this investigation was carried out retrospectively, and therefore limited our exploration of potential factors associated with cervical HIV-1 RNA shedding, such as plasma and genital antiretroviral drug concentrations. Furthermore, this was a cross-sectional study; therefore, we do not yet understand any treatment-related differences in cervical HIV-1 RNA shedding that may exist over time. We did not measure genital antiretroviral resistance in the 6 women with >1000 cervical copies/mL, and this is an important addition to future explorations of the class- or drug-specific relations between genital tract drug concentrations and effect on viral RNA shedding.
In summary, we have demonstrated that 44 (15%) of a cohort of 290 women on HAART with plasma HIV-1 RNA levels <500 copies/mL were shedding cervical HIV-1 RNA at a single time point. The risk of cervical HIV-1 RNA shedding was independently increased by use of NNRTI-based HAART versus PI-based HAART. The common occurrence of cervical viral RNA shedding in this cohort has implications for counseling women about the possibility of HIV transmission to infants or sexual partners or the spread of resistant virus from the sanctuary of the genital tract, even when plasma viral replication is effectively suppressed. The finding of more frequent cervical viral RNA shedding among women treated with an NNRTI-based antiretroviral regimen deserves further prospective investigation.
METHODS
Study Population
The Women's Interagency HIV Study (WIHS) has been previously described.6 Briefly, the WIHS is an ongoing cohort study conducted at 6 clinical sites across the United States (Bronx, NY; Brooklyn, NY; Washington, DC; Los Angeles and San Francisco Bay areas, CA; and Chicago, IL). The original WIHS cohort included a total of 2056 HIV-positive women enrolled from October 1, 1994, through November 15, 1995. An expansion cohort included 737 HIV-positive women enrolled from October 1, 2001, through September 30, 2002. The cross-sectional study reported here used data collected between April 1, 2000, and March 31, 2003 from 290 women from both cohorts. All HAART users with a cervical swab specimen available for HIV-1 RNA quantification with a concurrent plasma HIV-1 RNA level <500 copies/mL were eligible. A plasma viral RNA level of <50 copies/mL was defined in the standard fashion as undetectable, and a plasma viral RNA level from 50 to 500 copies/mL was defined as low. The upper threshold of 500 copies/mL was chosen to model the effect on cervical HIV-1 RNA shedding of a low level of plasma viral replication, or blips, above the lower limit of the standard plasma assay (400 copies/mL).
We classified HAART users in 2 groups: those who reported use of a PI-containing regimen (n = 179), defined by the use of at least 3 drugs comprising &8805;2 nucleoside analogue reverse transcriptase inhibitors (NRTIs) with &8805;1 PIs and no history of NNRTI use, including therapy to prevent mother-to-child transmission, and those who reported use of an NNRTI-containing regimen (n = 111), defined by the use of &8805;2 NRTIs with at least 1 NNRTI and no history of PI use.
Data Collection
We investigated a broad range of demographic and clinical characteristics as potential predictors of shedding. For this report, at each semiannual WIHS study visit, participants were queried about sexual activity since the last visit; the time of their last menses; use of tampons, intravaginal medications, or douches; illicit drug, cigarette, and hormonal contraceptive use; and symptoms associated with genital tract infections. Interval and current use of antiretroviral drugs was obtained by standardized self-report, assisted by use of photographic depictions of these drugs, labeled with brand, generic, and abbreviated names. Blood specimen testing included a complete blood cell count with lymphocyte subsets and plasma HIV-1 RNA level; cervical tract assessment included a vaginal swab for a pH and potassium hydroxide preparation (including an amine odor test and microscopy), visual inspection for blood, a saline preparation for microscopy (including the presence of red blood cells, Trichomonas vaginalis, and bacterial vaginosis by the Amstel definition), and 2 endocervical swabs for HIV-1 RNA quantification, followed by cervical cytology. All laboratories participate in the National Institute of Allergy and Infectious Diseases (NIAID) Division of Acquired Immune Deficiency Syndrome (DAIDS) Flow Cytometry Quality Assessment and Viral Quality Assurance (VQA) Programs. Testing at study visits for herpes, syphilis, gonorrhea, and Chlamydia infections was only performed if the history or physical examination was suggestive.
Cervical Swab Collection and Analysis
Cervical HIV-1 RNA shedding was measured using a Puritan (Guilford, ME) sterile Dacron polyester tip applicator 25-806-1PD swab as previously described.7,9 HIV-1 RNA quantification was done in our laboratory (University of Southern California, Los Angeles, CA) using isothermal nucleic acid sequence-based amplification (NucliSens; bioMerieux, Durham, NC, USA; lower limit of 25 copies/mL with 1-mL input) and 0.50 mL of the guanidine buffer in which each swab had been stored.7 This specimen collection approach reflects considerable prior research to determine the most sensitive method.1,8-14 Cervical HIV-1 RNA was reported in copies per milliliter of swab buffer.
Statistical Methods
The outcome of interest was the detection of cervical HIV-1 RNA shedding, defined as &8805;50 copies/mL in the cervical swab specimen. Logistic regression models with the logit link under a binomial distribution were used for univariate and multivariate analyses according to a stepwise modeling procedure.15 For forward selection at each step, involving each factor of interest, we used a likelihood ratio test (LRT) to 2 models: a reduced model with all factors identified from previous steps but without the factor of interest and a full model with all previously identified factors plus the factor of interest. For the initial step, the reduced model was an intercept-only model. We selected the factor that most significantly reduced the log likelihood provided that the P value for the LRT was <0.10. We also required that the P value for the Wald statistic for the given factor be <0.10. We used an alpha level of 0.15 for removal. The final model included only factors with Wald P values <0.05. All analyses were conducted using SAS (v9; SAS Institute, Cary, NC).
RESULTS
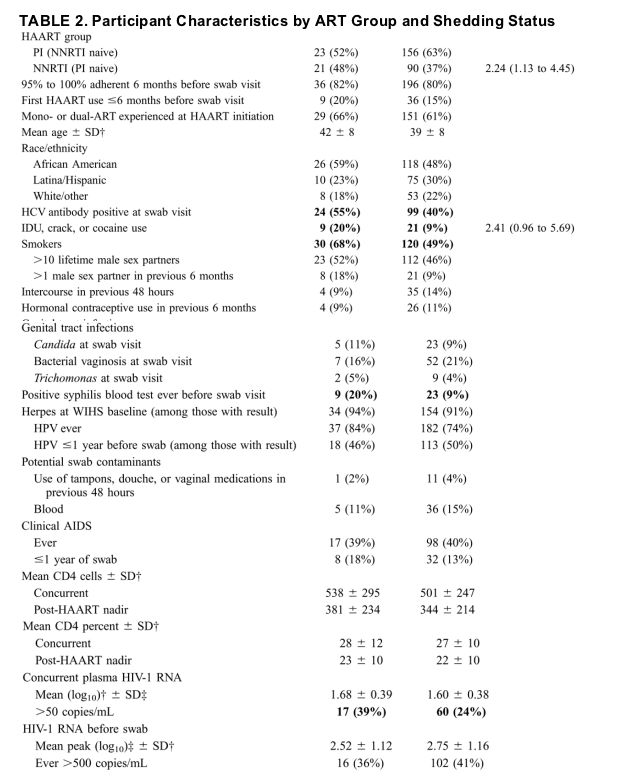
Cervical swab, history, and examination data were available from 290 women on HAART at the swab visit. We observed cervical HIV-1 RNA shedding in 44 (15%) women overall: 23 (13%) of the 179 PI users and 21 (19%) of the 111 NNRTI users. Among the 44 shedders, 6 (14%) were high shedders with >1000 copies/mL. Table 1 shows the distribution of shedders by drug and period of enrollment. Table 2 shows comparisons between nonshedders, and shedders. Shedders were more likely to have had more than 1 male sex partner in the previous 6 months, to have used crack cocaine or injected drugs, to have smoked tobacco, and to have 50 to 500 plasma HIV-1 RNA copies/mL at the swab collection visit. Shedders were also more likely to have a history of seropositivity for syphilis, but there was no association between shedding and a clinical assessment of genital tract inflammation or active infection with syphilis, herpes, gonorrhea, Chlamydia, or Trichomonas. There were no significant differences in reported rates of at least 95% adherence between women in the PI-naive NNRTI group or the NNRTI-naive PI group (P = 0.60).
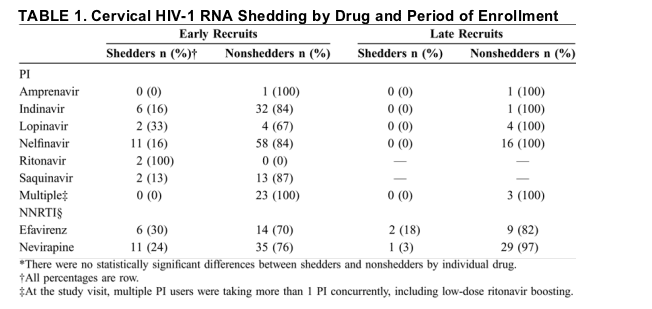
Original recruits were older (P < 0.0001), had a higher prevalence of hepatitis C virus (HCV; P < 0.0001), initiated HAART earlier (P < 0.0001), were more likely to use PIs (P < 0.0001), were more likely to have been exposed to mono- or dual-drug ART before HAART initiation (P < 0.0001), and were more likely to have had clinical AIDS within 1 year of the swab visit (P = 0.03) compared with the expansion recruits (data not shown). None of these factors were independently associated in the multivariate analysis with shedding, except for PI use.
Table 2 also shows the results of the stepwise multivariate analysis procedure. Adjusting for enrollment period, injection drug use, and NNRTI use were independently associated with shedding.
Six women (14% of all shedders and 2% of all women in the cohort) had cervical viral RNA levels >1000 copies/mL. This small sample, although interesting in the magnitude of shedding, lacks power to exclude differences from the whole cohort confidently. All 6 women were enrolled in the mid-1990s: 3 in the NNRTI group and 3 in the PI group. This was not a statistically significantly different distribution with respect to treatment than among shedders as a whole. As shown in Table 2, there were trends but no statistically significant differences between those with >1000 cervical RNA copies/mL and all shedders.
REFERENCES
1. Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593-1601.
[Context Link]
2. Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921-929.
[Context Link]
3. Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team [see comments]. N Engl J Med. 1999;341:385-393.
[Context Link]
4. Cao Y, Krogstad P, Korber BT, et al. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med. 1997;3:549-552.
[Context Link]
5. Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77:4-11.
[Context Link]
6. Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. Epidemiology. 1998;9:117-125.
[Context Link]
7. Bremer J, Nowicki M, Beckner S, et al. Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. Division of AIDS Treatment Research Initiative 009 Study Team. J Clin Microbiol. 2000;38:2665-2669.
[Context Link]
8. Al-Harthi L, Kovacs A, Coombs RW, et al. A menstrual cycle pattern for cytokine levels exists in HIV-positive women: implication for HIV vaginal and plasma shedding. AIDS. 2001;15:1535-1543.
[Context Link]
9. Baron P, Bremer J, Wasserman SS, et al. Detection and quantitation of human immunodeficiency virus type 1 in the female genital tract. The Division of AIDS Treatment Research Initiative 009 Study Group. J Clin Microbiol. 2000;38:3822-3824.
[Context Link]
10. Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184:1187-1191.
[Context Link]
11. Kemal KS, Foley B, Burger H, et al. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycosylation. Proc Natl Acad Sci USA. 2003;100:12972-12977.
[Context Link]
12. Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. AIDS. 2000;14:2101-2107.
[Context Link]
13. Hart CE, Lennox JL, Pratt-Palmore M, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871-882.
[Context Link]
14. Ellerbrock TV, Lennox JL, Clancy KA, et al. Cellular replication of human immunodeficiency virus type 1 occurs in vaginal secretions. J Infect Dis. 2001;184:28-36.
[Context Link]
15. Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons, New York, NY; 1989:106-118.
[Context Link]
16. Ioannidis JP, Abrams EJ, Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1000 copies/ml. J Infect Dis. 2001;183:539-545.
[Context Link]
17. Sullivan ST, Mandava U, Evans-Strickfaden T, et al. Diversity, divergence, and evolution of cell-free human immunodeficiency virus type 1 in vaginal secretions and blood of chronically infected women: associations with immune status 11704. J Virol. 2005;79:9799-9809.
[Context Link]
18. Overbaugh J, Anderson RJ, Ndinya-Achola JO, et al. Distinct but related human immunodeficiency virus type 1 variant populations in genital secretions and blood. AIDS Res Hum Retroviruses. 1996;12:107-115.
[Context Link]
19. Si-Mohamed A, Kazatchkine MD, Heard I, et al. Selection of drug-resistant variants in the female genital tract of human immunodeficiency virus type 1-infected women receiving antiretroviral therapy 12388. J Infect Dis. 2000;182:112-122.
[Context Link]
20. Fiore JR, Suligoi B, Saracino A, et al. Correlates of HIV-1 shedding in cervicovaginal secretions and effects of antiretroviral therapies. AIDS. 2003;17:2169-2176.
[Context Link]
21. Garcia-Bujalance S, Ruiz G, De Guevara CL, et al. Quantitation of human immunodeficiency virus type 1 RNA loads in cervicovaginal secretions in pregnant women and relationship between viral loads in the genital tract and blood. Eur J Clin Microbiol Infect Dis. 2004;23:111-115.
[Context Link]
22. Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J Acquir Immune Defic Syndr. 2004;37:1577-1580.
[Context Link]
23. Dumond J, Yeh R, Patterson K, et al. First dose and steady-state genital tract pharmacokinetics of ten antiretroviral drugs in HIV-infected women: implications for pre- and post-exposure prophylaxis [abstract 129 in poster abstracts]. Presented at: 13th Conference on Retroviruses and Opportunistic Infections; 2006; Denver.
[Context Link]
|
|
| |
| |
|
|
|