| |
Cirrhosis Is Associated with Low CD4+ T Cell Counts: Implications for HIV-Infected Patients with Liver Disease EDITORIAL
|
| |
| |
Clinical Infectious Diseases Feb 1,2007;44:438-44
Rajesh T. Gandhi
Infectious Diseases Unit and Partners AIDS Research Center, Massachusetts General Hospital, Boston
(See the article by McGovern et al. on pages 431-7)
".....the work of McGovern et al. [2] provides a fresh perspective on how cirrhosis affects absolute CD4+ T cell count....
....The findings of McGovern et al. [2] may also shed light on why different studies have had variable results when assessing the impact of HCV coinfection on the increase in CD4+ T cell count after initiation of antiretroviral therapy.... If the presence of cirrhosis in HIV-HCV-coinfected patients blunts the increase in CD4+ T cell count after the initiation of antiretroviral therapy, the observed differences between the results of these studies may be due to variability in the extent of underlying liver disease in the study populations. Examining the change in CD4+ T cell percentage after initiation of antiretroviral therapy in larger numbers of HIV-positive subjects (with and without HCV coinfection), which has been done in only a few published studies [3, 4, 17], may be illuminating....
... if CD4+ T cell percentage is a more accurate assessment of immune function in patients with cirrhosis, perhaps this measurement should be given greater weight when deciding whether to initiate antiretroviral therapy in these patients. For example, in a patient with HIV-HCV-coinfection, splenomegaly, a CD4+ T cell count of 250 cells/mm3, but a CD4+ T cell percentage of 30%, should antiretroviral therapy be initiated?....
"....Because CD4+ T cell percentage may be affected less by cirrhosis than absolute CD4+ T cell count, McGovern et al. [2] suggest that the former measurement may be more reliable in assessing the degree of immunosuppression in HIV-infected patients with advanced liver disease. This hypothesis could be tested by comparing the predictive value of absolute CD4+ T cell count with that of CD4+ T cell percentage for the development of AIDS-related conditions in HIV-infected patients with advanced liver disease...."
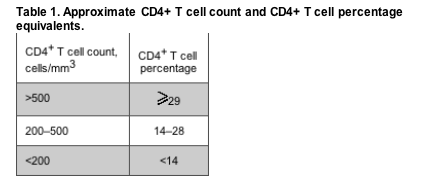
Low CD4+ T cell counts predict the development of AIDS-related conditions in HIV-infected patients. In laboratories in which the CD4+ T cell count is calculated, the absolute count is the product of 3 variables: total WBC count, the percentage of WBCs that are lymphocytes, and the percentage of lymphocytes that have cell surface expression of the CD4 receptor (the CD4+ T cell percentage). Absolute CD4+ T cell count and CD4+ T cell percentage are generally concordant (table 1). However, factors that perturb the number of WBCs or the percentage of lymphocytes may affect the absolute CD4+ T cell count without changing the CD4+ T cell percentage, thereby leading to discordance between the 2 values. McGovern et al. [2] have found that cirrhosis may be one factor that leads to low absolute CD4+ T cell counts despite relatively preserved CD4+ T cell percentages.
Sixty HIV-seronegative adults with cirrhosis that was assessed with histological analysis of the liver or clinical criteria underwent measurement of CD4+ T cell counts and percentages. In approximately one-half of the subjects, hepatitis C virus (HCV) infection was the cause of cirrhosis; the remainder had other causes of cirrhosis. Subjects who were currently using alcohol or medication that suppresses the CD4+ T cell count, such as corticosteroids, were excluded from the study. The main finding was that almost two-thirds of HIV-seronegative individuals with cirrhosis had abnormally low absolute CD4+ T cell counts (<550 cells/mm3); strikingly, 43% of the subjects had absolute CD4+ T cell counts of <350 cells/mm3, and 7% had absolute CD4+ T cell counts of <200 cells/mm3. These subjects with cirrhosis had statistically significantly lower CD4+ T cell counts than did >7600 HIV-negative historical control subjects (weighted group mean CD4+ T cell count, 492 cells/mm3 for patients with cirrhosis vs. 925 cells/mm3 for patients without cirrhosis; P < .001). Although the absolute CD4+ T cell count was low in many subjects with cirrhosis, the CD4+ T cell percentage was relatively preserved: of subjects who had low CD4+ T cell counts, 95% had normal CD4+ T cell percentages.
The discordance between absolute CD4+ T cell count and CD4+ T cell percentage observed in HIV-seronegative patients with cirrhosis may also occur in HIV-HCV-coinfected patients with liver disease. In a cohort of almost 2000 HIV-infected patients, HCV-seropositive patients had lower CD4+ T cell counts but higher CD4+ T cell percentages at study entry than did HCV-seronegative patients [3]. In a second study, HCV-seronegative and HCV-seropositive subjects with HIV had similar baseline CD4+ T cell counts, but the HCV-seropositive individuals had significantly higher CD4+ T cell percentages [4]. Both studies examined seroprevalent cohorts, so there may have been differences in the duration of HIV infection between the HCV-seropositive and HCV-seronegative groups. Nonetheless, the findings in these studies suggest that HIV-infected patients with HCV-induced liver disease may be more likely than HIV-infected subjects without HCV infection to have a discordance between the CD4+ T cell count and percentage, although it is clear that more studies are needed.
What is the mechanism of low absolute CD4+ T cell counts in HIV-negative subjects with cirrhosis? McGovern et al. [2] make a convincing case that this finding is related to portal hypertension and splenic sequestration of T cells. First, lower CD4+ T cell counts are associated with other cytopenias, such as lower WBC count, platelet count, hematocrit, and absolute CD8+ T cell count, as would be expected with sequestration of multiple cell types. Second, low CD4+ T cell counts are associated with the finding of splenomegaly at physical examination, which supports the hypothesis that pooling or destruction of T cells may be occurring in the enlarged spleen. Finally, low CD4+ T cell counts are associated with clinical markers of portal hypertension, such as low albumin level, hepatic encephalopathy, ascites, or esophageal varices. The authors observed no association between low CD4+ T cell count and the Childs-Pugh-Turcotte score (an assessment of the severity of liver disease), but their ability to find such an association may have been limited by the sample size of the study.
The hypothesis that cirrhosis and portal hypertension may lead to T cell sequestration and a low absolute CD4+ T cell count is physiologically plausible based on what we know about the effect of advanced liver disease on blood cell lines. Portal hypertension can lead to congestive splenomegaly; in patients with congestive splenomegaly due to portal hypertension, cytopenia of >1 cell line is common [5, 6]. These cytopenias may be the result of pooling or destruction of blood cells in the enlarged spleen. For example, thermally damaged, chromium-labeled RBCs disappear from circulation more rapidly in subjects who have cirrhosis than in individuals without liver disease [7, 8]. Larger spleen volume, as assessed with ultrasound or radionuclide imaging, is associated with lower WBC counts [9]. The fact that CD4+ T cell count tends to increase following a splenectomy also supports the idea that the spleen normally sequesters some of these cells from circulation. However, the degree of liver disease that is associated with a measurable T cell sequestration is not known. Does it occur only when a patient has clinical or histologic cirrhosis, as observed by McGovern et al. [2], or are earlier stages of liver disease also associated with lower CD4+ T cell counts? Further studies to address this question are warranted.
What, then, are the implications of the finding that a substantial proportion of HIV-seronegative subjects with cirrhosis have low absolute CD4+ T cell counts? If advanced liver disease leads to low CD4+ T cell counts in HIV-seronegative individuals, the same is likely to occur in HIV-seropositive patients. If this is the case, then the measurement of absolute CD4+ T cell count in HIV-infected patients with cirrhosis may not be as accurate in assessing the patient's degree of immunodeficiency as it is in HIV-infected patients without cirrhosis. Because CD4+ T cell percentage may be affected less by cirrhosis than absolute CD4+ T cell count, McGovern et al. [2] suggest that the former measurement may be more reliable in assessing the degree of immunosuppression in HIV-infected patients with advanced liver disease. This hypothesis could be tested by comparing the predictive value of absolute CD4+ T cell count with that of CD4+ T cell percentage for the development of AIDS-related conditions in HIV-infected patients with advanced liver disease.
The findings of McGovern et al. [2] may also shed light on why different studies have had variable results when assessing the impact of HCV coinfection on the increase in CD4+ T cell count after initiation of antiretroviral therapy. Although several studies have revealed that coinfected patients experience lower absolute CD4+ T cell count increases after initiating antiretroviral therapy than do HIV-monoinfected patients [10-15], other studies have not demonstrated such an effect [3, 16, 17]. A meta-analysis of these studies concluded that there was a small but statistically significant diminution in the increase in CD4+ T cell count in HCV-HIV-coinfected patients compared with that in HIV-monoinfected patients [18]; however, this meta-analysis focused on the change in absolute CD4+ cell count rather than the change in CD4+ T cell percentage. If the presence of cirrhosis in HIV-HCV-coinfected patients blunts the increase in CD4+ T cell count after the initiation of antiretroviral therapy, the observed differences between the results of these studies may be due to variability in the extent of underlying liver disease in the study populations. Examining the change in CD4+ T cell percentage after initiation of antiretroviral therapy in larger numbers of HIV-positive subjects (with and without HCV coinfection), which has been done in only a few published studies [3, 4, 17], may be illuminating.
The findings of McGovern et al. [2] also raise new questions that warrant scrutiny. Currently, in the United States, antiretroviral therapy for an HIV-infected patient is generally considered when the CD4+ T cell count decreases to <350 cells/mm3. However, if CD4+ T cell percentage is a more accurate assessment of immune function in patients with cirrhosis, perhaps this measurement should be given greater weight when deciding whether to initiate antiretroviral therapy in these patients. For example, in a patient with HIV-HCV-coinfection, splenomegaly, a CD4+ T cell count of 250 cells/mm3, but a CD4+ T cell percentage of 30%, should antiretroviral therapy be initiated? The answer to this question is not known, and until it is, most of us will continue to use absolute CD4+ T cell count values to guide treatment decisions. However, studies are needed to determine whether this is the right approach.
Finally, the findings of McGovern et al. [2] raise important pathophysiological questions about the interaction between HIV infection and liver disease. For example, previous studies have shown that, in HIV-HCV-coinfected patients, lower CD4+ T cell count is associated with more-severe liver fibrosis [19-23]. However, because absolute CD4+ T cell count is itself lowered by advanced liver disease, McGovern et al. [2] suggest that the examination of the association between CD4+ T cell percentage and hepatic fibrosis may better reveal the relationship between HIV-induced immunosuppression and liver disease.
In sum, the work of McGovern et al. [2] provides a fresh perspective on how cirrhosis affects absolute CD4+ T cell count. Future studies will be needed to determine the full implications of these important findings for HIV-infected patients with liver disease.
References
1. John G. Bartlett, Joel E. Gallant, eds. Medical Management of HIV Infection. Baltimore: Johns Hopkins University, Division of Infectious Diseases and AIDS Service, 2003:19. First citation in article
2. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis 2007; 44:431-7 (in this issue). First citation in article
3. Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA 2002; 288:199-206. First citation in article | PubMed | CrossRef
4. Braitstein P, Zala C, Yip B, et al. Immunologic response to antiretroviral therapy in hepatitis C virus-coinfected adults in a population-based HIV/AIDS treatment program. J Infect Dis 2006; 193:259-68. First citation in article | Full Text | PubMed
5. Eichner ER. Splenic function: normal, too much and too little. Am J Med 1979; 66:311-20. First citation in article | PubMed | CrossRef
6. Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol 2000; 95:2936-9. First citation in article | PubMed | CrossRef
7. Holzbach RT, Clark RE, Shipley RA, Kent WB 3rd, Lindsay GE. Evaluation of spleen size by radioactive scanning. J Lab Clin Med 1962; 60:902-13. First citation in article | PubMed
8. Wagner HN Jr, Razzak MA, Gaertner RA, Caine WP Jr, Feagin OT. Removal of erythrocytes from the circulation. Arch Intern Med 1962; 110:90-7. First citation in article | PubMed
9. Shah SH, Hayes PC, Allan PL, Nicoll J, Finlayson ND. Measurement of spleen size and its relation to hypersplenism and portal hemodynamics in portal hypertension due to hepatic cirrhosis. Am J Gastroenterol 1996; 91:2580-3. First citation in article | PubMed
10. Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 2000; 356:1800-5. First citation in article | PubMed | CrossRef
11. Lincoln D, Petoumenos K, Dore GJ. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med 2003; 4:241-9. First citation in article | PubMed | CrossRef
12. Rancinan C, Neau D, Saves M, et al. Is hepatitis C virus co-infection associated with survival in HIV-infected patients treated by combination antiretroviral therapy? AIDS 2002; 16:1357-62. First citation in article | PubMed | CrossRef
13. Macias J, Pineda JA, Lozano F, et al. Impaired recovery of CD4+ cell counts following highly active antiretroviral therapy in drug-naive patients coinfected with human immunodeficiency virus and hepatitis C virus. Eur J Clin Microbiol Infect Dis 2003; 22:675-80. First citation in article | PubMed | CrossRef
14. Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2003; 33:365-72. First citation in article | PubMed
15. De Luca A, Bugarini R, Lepri AC, et al. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med 2002; 162:2125-32. First citation in article | PubMed | CrossRef
16. Chung RT, Evans SR, Yang Y, et al. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS 2002; 16:1915-23. First citation in article | PubMed | CrossRef
17. Rockstroh JK, Mocroft A, Soriano V, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis 2005; 192:992-1002. First citation in article | Full Text | PubMed
18. Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis 2005; 41:713-20. First citation in article | Full Text | PubMed
19. Rockstroh JK, Spengler U, Sudhop T, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol 1996; 91:2563-8. First citation in article | PubMed
20. Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut 2003; 52:1035-40. First citation in article | PubMed | CrossRef
21. Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology 2001; 34:1193-9. First citation in article | PubMed | CrossRef
22. Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999; 30:1054-8. First citation in article | PubMed | CrossRef
23. Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003; 36:491-8. First citation in article | Full Text | PubMed
|
|
| |
| |
|
|
|