| |
Cirrhosis is Associated with Low CD4 Counts
|
| |
| |
"The Impact of Cirrhosis on CD4+ T Cell Counts in HIV-Seronegative Patients"
Clinical Infectious Diseases Feb 1, 2007;44:431-437
Barbara H. McGovern,1 Yoav Golan,1 Marvin Lopez,2 Daniel Pratt,2 Angela Lawton,1 Grayson Moore,1 Mark Epstein,2 and Tamsin A. Knox2
Divisions of 1Geographic Medicine and Infectious Diseases and 2Gastroenterology, Tufts-New England Medical Center, Boston, Massachusetts
(See the editorial commentary by Gandhi)
AUTHOR DISCUSSION
Our study results highlight a previously unreported association between low absolute CD4+ T cell counts and cirrhosis in the absence of HIV infection. Sixty-five percent of our study cohort of cirrhotic patients had abnormally low CD4+ T cell counts (<550 cells/mm3); 43% had CD4+ T cell counts <350 cells/mm3. In contrast, 95% of those with abnormal CD4+ T cell counts had normal CD4+ T cell percentages. Low CD4+ T cell counts in these patients were not associated with demographic characteristics or with the etiology of the underlying liver disease.
We believe that low CD4+ T cell counts are the result of global sequestration of blood cell lines related to portal hypertension. This hypothesis is supported by the strong association between low CD4+ T cell counts and splenomegaly, leukopenia, and severe thrombocytopenia-conditions that are commonly seen in patients with cirrhosis, regardless of etiology [25]. These novel findings have far-reaching significance, because approximately one-third of all HIV-infected patients worldwide have chronic viral hepatitis and are at risk for developing advanced liver disease [26, 27].
In studies comparing HIV-HCV-coinfected patients to patients with HCV alone, low CD4+ T cell counts have been linked to faster liver fibrosis progression rates in patients with concomitant HIV infection [1-5] . The authors concluded that HIV-associated immunodeficiency contributes to accelerated liver disease progression. These studies have inferred that control patients with HCV alone had normal CD4+ T cell counts. However, we have demonstrated that cirrhosis itself can lead to low absolute CD4+ T cell counts, in the absence of HIV infection. Future studies comparing HCV-related fibrosis progression in HIV-seropositive patients with HIV-seronegative patients will need to measure CD4+ T cell counts and percentages in both groups to accurately study the strength of the association between HIV-associated immunosuppression and cirrhosis.
Our study results also have implications for the observation that patients with HIV-HCV coinfection have a "blunted" immune response to antiretroviral therapy. A recent metaanalysis of 8 trials involving 6216 patients demonstrated that HIV-HCV-coinfected patients had a mean increase in the CD4+ T cell count that was 33 cells/mm3 less than the mean increase for HIV-infected patients without HCV infection [28]. Whether splenic sequestration of T cells may play a contributory role in this phenomenon is unknown.
Accurate assessment of a patient's immunologic status is also critical when evaluating candidacy for liver transplantation in the HIV-infected patient [29]. However, absolute CD4+ T cell counts may not fully reflect the stage of HIV infection in the patient with advanced liver disease. We suggest that the use of CD4+ T cell percentages should be incorporated into liver transplantation protocols to assure a more precise assessment of the host's immunologic status.
Finally, some studies of HIV-infected patients have demonstrated that use of CD4+ T cell percentages adds useful prognostic information in certain patient subgroups when compared with absolute T cell counts [30, 31]. Whether CD4+ T cell percentages may also help in assessing the immune status of HIV-infected patients with liver disease is unknown, but should be evaluated in future clinical trials.
A potential limitation of this study is related to the lack of a contemporary control group consisting of healthy adults. However, the distribution of absolute T cell counts and percentages of T cell subsets were readily available from multiple studies that enrolled healthy adults, allowing a comparison of our findings with validated results from historic controls. The small differences in CD4+ T cell counts observed among HIV-seronegative adult patients from different countries, races, and sexes cannot account for the substantial differences observed between CD4+ T cell distributions in our cohort and the historic controls. Furthermore, biologic variability in T cell assessments (ranging from 6%-8% in HIV-seronegative patients) [32, 33] does not explain the significant abnormalities of CD4+ T cell counts that were noted in our study.
In summary, cirrhosis is associated with low CD4+ T cell counts in the absence of HIV infection. Future studies of fibrosis progression rates need to evaluate CD4+ T cell counts and percentages in all HCV patients, regardless of HIV serostatus, to accurately define the association between immunosuppression and liver disease. Liver transplant protocols for HIV-infected patients should incorporate the use of CD4+ T cell percentages to better assess the candidate's immunologic status.
ABSTRACT
Background. Studies of the progression liver fibrosis in human immunodeficiency virus (HIV) and hepatitis C virus-coinfected patients suggest that cirrhosis is associated with immunosuppression, as measured by low absolute CD4+ T cell counts. However, we hypothesized that, in patients with advanced liver disease, low CD4+ T cell counts may occur secondary to portal hypertension and splenic sequestration, regardless of the presence or absence of HIV infection.
Methods. Sixty HIV-seronegative outpatients with cirrhosis were enrolled during the period 2001-2003 in a prospective, cross-sectional study of the association between liver disease and CD4+ T cell counts and percentages. Demographic characteristics, liver disease-related characteristics, and laboratory results-including CD4+ T cell parameters-were collected.
Results.
A total of 39 patients (65%) had a low CD4+ T cell count; 26 patients (43%) and 4 patients (7%) had CD4+ T cell counts <350 and <200 cells/mm3, respectively. Abnormal CD4+ T cell counts were associated with splenomegaly (P = .03), thrombocytopenia (P = .002), and leukopenia (P < .001).
The percentage of CD4+ T cells was normal in 95% of patients who had a low absolute CD4+ T cell count. CD4+ T cell counts were significantly lower among cirrhotic patients than among 7638 HIV-seronegative historic control subjects without liver disease.
Conclusions. Cirrhosis is associated with low CD4+ T cell counts in the absence of HIV infection. Discordance between low absolute CD4+ T cell counts and normal CD4+ T cell percentages may be attributable to portal hypertension and splenic sequestration. Our findings have significant implications for the use and interpretation of absolute CD4+ T cell counts in HIV-infected patients with advanced liver disease.
Background
In natural history studies of hepatitis C virus (HCV)-infected patients, accelerated rates of fibrosis progression have been associated with an HIV-seropositive status, especially in the setting of low CD4+ T cell counts. Five studies that compared HIV-HCV-coinfected patients to subjects with HCV infection alone found that progression to cirrhosis was associated with immunosuppression [1-5]. In these studies, CD4+ T cell counts in the HIV-seronegative-HCV-infected control groups were assumed to be normal. It was concluded that advanced HIV infection, as reflected by low CD4+ T cell counts, was associated with cirrhosis.
Whether cirrhosis itself can lead to depletion of absolute CD4+ T cell counts is unknown, but it is plausible. Portal hypertension is associated with leukopenia and thrombocytopenia through splenic sequestration; thus, it is possible that T-cell sequestration may occur as well. In addition, we have observed that many of our HIV-infected patients who have advanced liver disease (secondary to chronic viral hepatitis) have discordant patterns of low CD4+ T cell counts with normal CD4+ T cell percentages. This clinical observation suggests that an alternate mechanism may explain the decrease in CD4+ T cells in patients with underlying liver disease.
To test the hypothesis that low CD4+ T cell counts may occur with advanced liver disease, regardless of HIV status, we designed a cross-sectional survey of HIV-seronegative cirrhotic patients with the following aims: (1) to describe the distribution of CD4+ T cell counts in patients with cirrhosis and the relation of this distribution to CD4+ T cell percentage, (2) to examine whether viral versus nonviral etiologies of liver disease have a differential effect on absolute CD4+ T cell counts, (3) to examine the association between abnormally low CD4+ T cell counts and demographics and markers of liver dysfunction and portal hypertension, and (4) to compare the distribution of CD4+ T cell counts in patients with cirrhosis to those of historic control subjects who do not have liver disease.
METHODS
Patient population. In a prospective, cross-sectional study, consecutive adult patients with advanced liver disease of various etiologies who were observed for development of cirrhosis at 2 gastroenterology clinics in Boston were recruited during the period 2001-2003. For inclusion in the study, patients had to be HIV seronegative and had to have cirrhosis, as determined by a liver biopsy or by clinical criteria (e.g., splenomegaly, esophageal varices, ascites, and jaundice). The presence or absence of varices was determined from the results of endoscopy, performed either for bleeding or as a screening examination. HIV status was established prior to enrollment as part of the routine pretransplant evaluation criteria or by patient request. Exclusion criteria included fulminant liver failure, active bacterial infection, use of immunosuppressive therapy (including corticosteroids), consumption of >2 alcoholic drinks per day for men or 1 drink a day for women [6], and history of splenectomy. The goal of recruitment was to prospectively enroll 60 HIV-seronegative patients with viral and nonviral etiologies of liver disease. The Institutional Review Board of Tufts-New England Medical Center (Boston, MA) approved the research protocol. All study participants provided written informed consent prior to study enrollment.
Clinical and laboratory data. Two physicians and a research nurse gathered data from patients' medical charts and the computerized database for documentation onto a structured questionnaire. The following data were collected: demographic characteristics (age, sex, and race), etiology of liver disease (hepatitis B or C, alcohol related, cryptogenic cirrhosis, primary biliary cirrhosis, sclerosing cholangitis, autoimmune hepatitis, a-1 antitrypsin, and steatohepatitis), physical findings at the time of enrollment (including the presence of splenomegaly, as documented by the attending hepatologist), and laboratory data.
Definitions and tests. The severity of liver disease was assessed using Child-Pugh-Turcotte (CPT) scores [7]. Classification of CPT scores was assigned by the attending hepatologist on the basis of established criteria that use laboratory parameters of synthetic function (bilirubin, prothrombin, and serum albumin) and clinical assessment (presence of encephalopathy or ascites). CPT scores range from 5 to 15 points, with higher scores indicating more severe liver dysfunction.
Whole-blood samples were collected in EDTA anticoagulant for measurement of absolute CD4+ T cell counts and CD4+ T cell percentages. Samples were kept at room temperature and processed within the same day of collection, as recommended by the Centers for Disease Control and Prevention (CDC) [8]. Flow cytometry was performed on a Beckman-Coulter Epics XL (Beckman-Coulter) in the clinical laboratory of Tufts-New England Medical Center, which processes >200 clinical samples for flow cytometry on a monthly basis. Controls are set up daily and samples are processed at room temperature within 24 h of receipt, as recommended by CDC guidelines [8, 9]. The accuracy of the assay is also confirmed by surveys from the College of American Pathologists (CAP), which are performed every 4 months [9]. A CD4+ T cell count <550 cells/mm3 and a CD4+ T cell percentage <30% were defined as abnormal in the laboratory where the flow cytometry was performed.
Historical controls. To establish a group of historical controls, we used published reports of CD4+ T cell counts in HIV-seronegative persons. The distribution of CD4+ T cell counts and percentages in HIV-seronegative persons is known, validated, and used as references in flow cytometry assays [9]. We searched the Medline database to identify all studies reporting the distribution of CD4+ T cell counts in HIV-seronegative persons (date of last search, 1 November 2005). Search terms included "lymphocyte subsets," "T-cell subsets," and "CD4+ T cells." Because CD4+ T cell counts are affected by age (they are higher in children), but are only minimally affected by race or geography, we excluded studies that enrolled persons <18 years of age but not studies that included patients in countries other than the United States. We also excluded studies that (1) did not report the mean or variance of the CD4+ T cell distribution, (2) did not use flow cytometry as the testing method, and (3) only included patients with a specific illness or liver disease.
Using a random-effect model, we calculated a grouped, weighted mean and SE based on the sample size, mean, and SD of each of the reports. We subjected the grouped mean and the SE, as well as the mean and SE from our group of patients with advanced liver disease, to a t test formula. This allowed us to calculate a t statistic and to obtain a P value for the likelihood that the distribution of CD4+ T cell counts observed in our study is similar to that reported in historic controls.
Analysis. We examined the association between 2 levels of abnormal CD4+ T cell counts, <550 cells/mm3 and <350 cells/mm3, and patients' demographic characteristics and characteristics of liver disease in univariate analyses. The cutoff of 550 cells/mm3 was chosen because any result below this number was considered abnormal in the validation assays for our flow cytometry test. The cutoff of 350 cells/mm3 was selected because this threshold is often used for the initiation of antiretroviral therapy in HIV-infected patients [10]. The 2 test was used for binary variables and Student's t test was used for continuous variables. A P value <.05 determined statistical significance. All analyses were performed using SAS software, version 8.1 (SAS Institute).
RESULTS
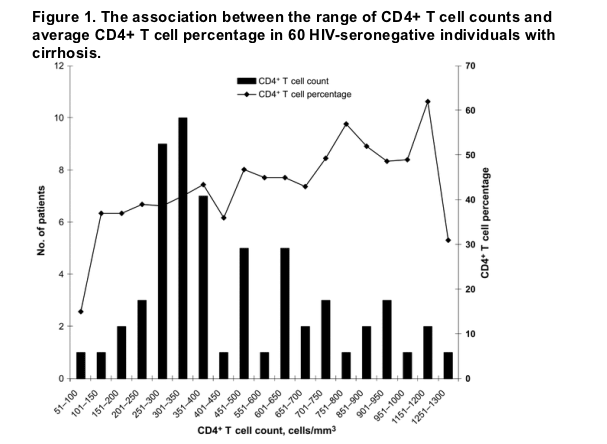
Demographic characteristics, etiology of liver disease, and CPT score class. Sixty patients with a history of cirrhosis were enrolled in our study. Most patients were white (54 patients [90%]) and male (38 patients [63%]) (table 1). The mean age was 50 years. Thirty-two patients had viral etiologies of liver disease, including hepatitis C (31 patients) and hepatitis B (1 patient). The remaining 28 patients had nonviral etiologies, including alcoholic cirrhosis (12 patients), primary biliary cirrhosis (4 patients), primary sclerosing cholangitis (5 patients), cryptogenic cirrhosis (4 patients), steatohepatitis (1 patient), autoimmune hepatitis (1 patient), and -1 antitrypsin deficiency (1 patient). CPT scores were as follows: class A (21 patients), class B (24 patients), and class C (15 patients).
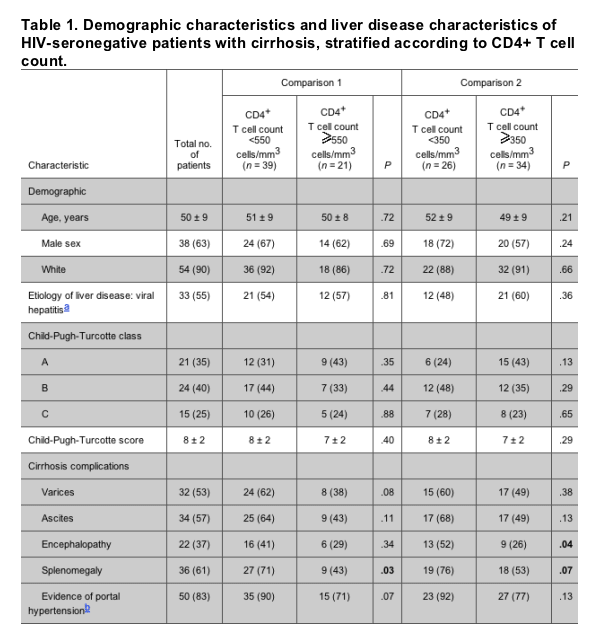
Distribution of CD4+ T cell counts and its relation to CD4+ T cell percentage. The distribution of CD4+ T cell counts was approximately normal (figure 1). The range of CD4+ T cell counts was 52-1269 cells/mm3. The mean, median, and SD were 492 cells/mm3, 384 cells/mm3, and 278 cells/mm3, respectively. Thirty-nine (65%) of the study patients had abnormal CD4+ T cell counts; 43% (n = 26) and 7% (n = 4) had counts <350 cells/mm3 and 200 cells/mm3, respectively.
The range of CD4+ T cell percentages was 15%-66%. The mean, median, and SD were 43%, 45%, and 10%, respectively. The mean CD4+/CD8+ T cell ratio was 2.8. Of patients with low CD4+ T cell counts, 57 (95%) had a normal CD4+ T cell percentage.
Risk factors for low CD4+ T cell counts in patients with advanced liver disease. Patients with abnormal CD4+ T cell counts (<550 cells/mm3 or <350 cells/mm3) did not differ from patients with normal CD4+ T cell counts in terms of age, sex, or race (table 1). Furthermore, neither the etiology of the underlying liver disease (viral vs. nonviral) nor CPT score differentiated the patient groups. An increased CPT score was not associated with a decreased CD4+ T cell count (r2 = .02; P = .25).
Abnormal CD4+ T cell counts (<550 cells/mm3 or <350 cells/mm3) were associated with splenomegaly (P = .03) and decreases in WBC count (P = .014), platelet count (P = .002), absolute CD8+ T cell count (P = .02), and CD4+ T cell percentage (P = .002), compared with normal CD4+ T cell counts (table 2). Lower albumin levels and occurrence of hepatic encephalopathy were associated with a CD4+ T cell count of <350 cells/mm3 (P values of .03 and .04, respectively). The ratio of CD4+ T cells to CD8+ T cells and the level of transaminases, total bilirubin, or international normalized ratio did not correlate with the presence of low CD4+ T cell counts.
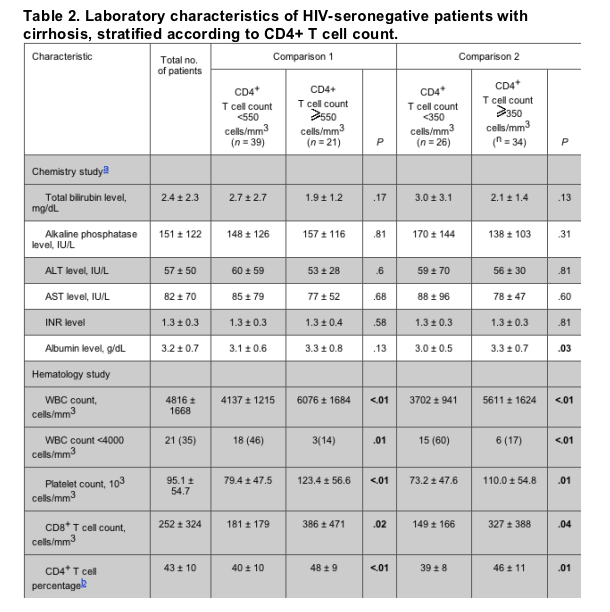
The association between low CD4+ T cell counts and clinical markers of portal hypertension was also explored. When analyzed as a continuous variable, lower CD4+ T cell counts were associated with the occurrence of esophageal varices (P = .02), splenomegaly (P = .04), and any manifestation of portal hypertension (defined as having evidence for at least 1 of the following: splenomegaly, encephalopathy, ascites, or esophageal varices; P < .01). Low absolute CD4+ T cell counts were also associated with lower levels of the following laboratory parameters: albumin (P = .03), leukocytes (P < .01), platelets (P < .01), CD8+ T cells (P < .01), and hematocrit (P = .03).
Comparison of the distribution of CD4+ T cell counts in patients with advanced liver disease with historic control subjects. In a search of the literature, we identified 15 articles that qualified for inclusion of historic control subjects without cirrhosis (figure 2) [11-24]. These reports included 7638 HIV-seronegative adults from multiple countries. The weighted grouped mean of the CD4+ T cell count in historical controls was 925 cells/mm3, and the grouped SE was 3 cells/mm3. In our 60 cirrhotic subjects, the mean CD4+ T cell count was 492 cells/mm3, with a SE of 36 cells/mm3. The difference in CD4+ T cell counts between that of the group of patients with advanced liver disease and that of historic control subjects (without cirrhosis) was statistically significant at P < .001.
References
1. Benhamou Y, Bochet M, DiMartino V, Charlotte F, Azria F, Coutellier A. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. Hepatology 1999; 30:1054-8. First citation in article | PubMed | CrossRef
2. Martinez-Sierra C, Arizcorreta A, Diaz F, Roldan R. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003; 36:491-8. First citation in article | Full Text | PubMed
3. Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut 2003; 52:1035-40. First citation in article | PubMed | CrossRef
4. Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology 2001; 34:1193-9. First citation in article | PubMed | CrossRef
5. Rockstroh JK, Spengler U, Sudhop T, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol 1996; 91:2563-8. First citation in article | PubMed | CrossRef
6. Pol S, Artru P, Thepot V, Berthelot P, Nalpas B. Improvement of the CD4 cell count after alcohol withdrawal in HIV-positive alcoholic patients. AIDS 1996; 10:1293-4. First citation in article | PubMed
7. Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Brit J Surg 1973; 60:646-9. First citation in article | PubMed | CrossRef
8. Mandy FF, Nicolson JK, McDougal JS. Guidelines for performing single-platform antibody CD4+ T-cell determinations with CD45 gating in persons infected with human immunodeficiency virus. Centers for Disease Control and Prevention. MMWR Recomm Rep 2003; 52(RR-2):1-13. First citation in article | CrossRef
9. Scornik JC, Bray RA, Pollack MS, et al. Multicenter evaluation of the flow cytometry T-cell crossmatch: results from the American Society of Histocompatibility and Immunogenetics-College of American Pathologists proficiency testing program. Transplantation 1997; 63:1440-5. First citation in article | PubMed | CrossRef
10. Yeni PG, Hammer S, Hirsch M, et al. Treatment of adult HIV infection: 2004 recommendations of the International AIDS Society-USA panel. JAMA 2004; 292:251-65. First citation in article | PubMed | CrossRef
11. Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol 2004; 72:203-12. First citation in article | PubMed | CrossRef
12. Uppal SS, Verma S, Dhot PS. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Clinical Cytometry 2003; 52:32-6. First citation in article | PubMed | CrossRef
13. Tsegaye A, Mesele T, Tilahun T, et al. Immunohematological reference ranges for adult Ethiopians. Clin Diagn Lab Immunol 1999; 6:410-4. First citation in article | PubMed
14. Levin A, Brubaker G, Shao JS, et al. Determination of T-lymphocyte subsets on site in rural Tanzania: results in HIV-infected and non-infected individuals. Int J STD AIDS 1996; 7:288-91. First citation in article | PubMed | CrossRef
15. Menard D, Mandeng MJ, Tothy MB, Kelembho EK, Gresenguet G, Talarmin A. Immunohematological reference ranges for adults from the Central African Republic. Clin Diagn Lab Immunol 2003; 10:443-5. First citation in article | PubMed | CrossRef
16. Tugume SB, Piwowar EM, Lutalo T, et al. Hematological reference ranges among healthy Ugandans. Clin Diagn Lab Immunol 1995; 2:233-5. First citation in article | PubMed
17. Kam KM, Leung WL, Kwok MY, Hung MY, Lee SS, Mak WP. Lymphocyte subpopulation reference ranges for monitoring human immunodeficiency vrius-infected Chinese adults. Clin Diagn Lab Immunol 1996; 3:326-30. First citation in article | PubMed
18. Urassa WK, Mbena EM, Swai AB, Gaines H, Mhalu FS, Biberfeld G. Lymphocyte subset enumeration in HIV seronegative and HIV-1 seropositive adults in Dar es Salaam, Tanzania: determination of reference values in males and females and comparison of two flow cytometric methods. J Immunol Methods. 2003; 277:65-74. First citation in article | CrossRef
19. Yaman A, Cetiner S, Kibar F, Tasova Y, Seydaoglu G, Dundar IH. Reference ranges of lymphocyte subsets of healthy adults in Turkey. Med Princ Pract 2005; 14:189-93. First citation in article | PubMed | CrossRef
20. Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subsets reference range for an Asian population by single-platform flow-cytometry: influence of age, sex and race and comparison with other published studies. Clin Diagn Lab Immunol 2004; 11:168-73. First citation in article | PubMed | CrossRef
21. Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A. Lymphocyte subsets' reference ranges in an age- and gender-balanced population of 100 heatlhy adults-a monocentric German study. Clin Immunol 2005; 116:192-7. First citation in article | PubMed | CrossRef
22. Bofil M, Janossy G, Lee C, et al. Laboratory control values for CD4 and CD8 T lymphocytes. Clin Exp Immunol 1992; 88:243-52. First citation in article | PubMed
23. Aina O, Dadik J, Charurat M, et al. Immunohematological reference ranges for adult Ethiopians. Clin Diagn Lab Immunol 2005; 12:525-30. First citation in article | PubMed | CrossRef
24. Giorgi JV, Cheng HL, Margolick J, Bauer KD, Ferbas J, Waxdal M. Quality control in the flow cytometric measurement of T-lymphocyte subsets: the muticenter AIDS cohort study experience. The Multicenter AIDS Cohort Study Group. Clin Immunol Immunopathol 1990; 55:173-86. First citation in article | PubMed | CrossRef
25. Eyster ME, Whitehurst DA, Catalano PM, et al. Long-term follow-up of hemophiliacs with lymphocytopenia or thrombocytopenia. Blood 1985; 66:1317-20. First citation in article | PubMed
26. Sherman K, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis 2002; 34:831-7. First citation in article | Full Text | PubMed
27. Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS 2005; 19:593-601. First citation in article | PubMed | CrossRef
28. Miller M, Haley C, Koziel M, Rowley C. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis 2005; 41:713. First citation in article | Full Text | PubMed
29. Neff GW, Bonham A, Tzakis A, Ragni M. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transplantation 2003; 9:239-47. First citation in article | PubMed | CrossRef
30. Hulgan T, Raffanti S, Kheshti A, et al. CD4 lymphocyte percentage predicts disease progression in HIV-infected patients initiating highly active antiretroviral therapy with CD4 lymphocyte counts >350 lymphocytes/mm3. J Infect Dis 2005; 192:950-7. First citation in article | Full Text | PubMed
31. Mulcahy F, Wallace E, Woods S, et al. CD4 counts in pregnancy do not accurately reflect the need for long-term HAART [abstract 704B]. In: Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections (Denver, CO). Alexandria, VA: Foundation for Retrovirology and Human Health, 2006. First citation in article
32. Puro V, Ippolito G. Effect of antiretroviral agents on T-lymphocyte subset counts in healthy HIV-negative individuals. J Acquir Immune Defic Syndr 2000; 24:440-3. First citation in article | PubMed
33. Centers for Disease Control and Prevention. Guidelines for the performance of CD4+ T-cell determinations in persons with human immunodeficiency virus infection. MMWR Recomm Rep 1992; 41(RR-8):1-17. First citation in article | CrossRef
|
|
| |
| |
|
|
|