| |
HIV, Diabetes & Impaired Glucose Tolerance: prevalence, diagnosing, treatment
|
| |
| |
Written By Jules Levin
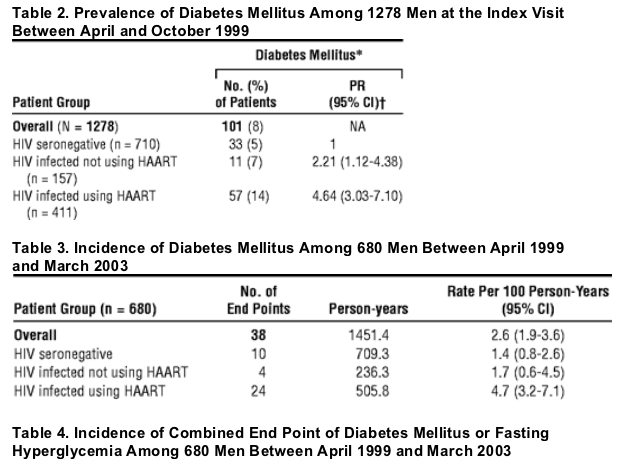
In the MACS cohort, the 4-year risk for diabetes (DM) of 10% is higher than previous estimates and supports the importance of regular screening for hyperglycemia among HIV-infected persons.
Some investigators have proposed that IR in HIV-infected persons may be attributable to specific HIV therapies such as indinavir, increased accumulation of visceral fat and decreased peripheral fat, cytokines, and even HIV infection per se. Greater waist circumference, triglycerides, age, and alanine aminotransferase have been associated with worse IR.
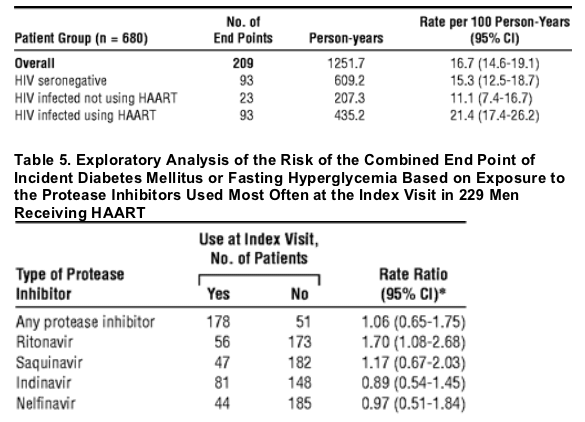
In the Multicenter AIDS Cohort Study, during a 4-year follow-up period in the MACS, 24 (10%) of 229 HIV-infected subjects receiving HAART developed DM compared with 10 (3%) of 361 HIV-seronegative men. After adjustment for BMI and age, this difference represents a greater than 4-fold increase in the risk of incident DM among HIV-infected subjects receiving HAART. The HIV-infected men not using HAART had an increased risk of prevalent DM relative to the HIV-seronegative group after adjustment for age and BMI. (Arch Intern Med. 2005;165:1179-1184. Tables 2, 3 and 4 below). Of the 680 men in the incidence analysis, 209 developed the combined end point of DM or hyperglycemia (Table 4), yielding an adjusted RR of 1.64 (95% CI, 1.21-2.33) in the HIV-infected group using HAART compared with the HIV-seronegative group. The incidence of the combined end point of DM or hyperglycemia based on the use of specific PIs is given in Table 5. Only ritonavir was significantly associated with an increased rate of the combined end point (RR = 1.70; 95% CI, 1.08-2.68) relative to men not using ritonavir, adjusting for age, BMI, nadir CD4 cell count, and cumulative use of nucleoside reverse transcriptase inhibitors (NRTIs) and NNRTIs. Classification of exposure to the PIs as "ever or never" use did not change our inferences (data not shown).
Since the advent of highly active antiretroviral therapy (HAART) in the mid-1990s, abnormalities in glucose homeostasis have been reported with increasing frequency in persons infected with human immunodeficiency virus (HIV).1-4 Impaired glucose tolerance was observed in 25 (35%) of 71 HIV-infected patients using HAART.6 Prevalence estimates of diabetes mellitus (DM) are lower. In a cross-sectional study, 28 (6%) of 493 HIV-infected patients had DM.7
Among HIV-infected adults receiving medical care in an urban clinic, we observed an increased prevalence of hyperglycemia among persons with HCV coinfection compared with those without HCV infection prior to the initiation of HAART. Moreover, both HCV coinfection and PI use appeared to increase the risk of new-onset hyperglycemia during HAART. Both of these effects were independent of other risk factors of hyperglycemia including age, race, and body weight. The detection of an increased prevalence of hyperglycemia among HIV-infected persons with HCV coinfection compared with those without HCV infection is consistent with nearly 30 other reports that have suggested an association between HCV infection and diabetes. These reports have documented that the prevalence of diabetes among persons with HCV infection ranges from 25-50%. 12-14 Our study extends these findings by detecting a similar positive association of HCV and hyperglycemia in another group of persons, HIV/HCV-coinfected persons. (The Effect of HAART and HCV Infection on the Development of Hyperglycemia Among HIV-Infected Persons; JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 33(5) 15 August 2003 pp 577-584; Mehta, Shruti H.*; Moore, Richard D. ; Thomas, David L.* ; Chaisson, Richard E. ; Sulkowski, Mark S. , *Department of Epidemiology and Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland).
DISORDERS OF GLUCOSE METABOLISM IN HIV
Characteristics and Risk Factors
A spectrum of disorders of glucose metabolism has been associated with HIV infection and antiretroviral therapy. IR occurs when the target tissues of insulin action fail to respond appropriately to insulin, resulting in increased pancreatic insulin production. Impaired glucose tolerance is an elevated blood sugar level of 140-199 mg/dL 2 h after receipt of a 75-g loading dose of glucose during an oral glucose tolerance test [62]. Impaired fasting glucose tolerance occurs when the fasting blood sugar level is in the 100-125-mg/dL range. The presence of impaired glucose tolerance or impaired fasting glucose tolerance suggests that IR may be present. DM is diagnosed when the fasting blood sugar level is >126 mg/dL, or the 2-h oral glucose tolerance test glucose level is >200 mg/dL and is confirmed by additional testing, or when a patient has symptoms of DM (frequent urination, thirst, blurred vision, or weight loss) in the setting of a blood glucose level >200 mg/dL.
Risk factors for the development of disorders of glucose metabolism include obesity, lipoatrophy, use of most PIs, NRTI exposure (particularly stavudine), older age, family history of DM, nonwhite race, and possibly hepatitis C virus coinfection [63, 64]. Other data suggest that traditional risk factors for IR are more important than treatment-related factors [65]. Use of niacin, growth hormone, corticosteroids, and antipsychotics may also contribute to hyperglycemia.
Screening and Diagnosis
-- A fasting (>8 h) blood glucose level should be checked before initiation of HIV therapy and should be monitored every 3-6 months for patients with changes in treatment regimen or who have significant risk factors for IR.
-- For patients with impaired glucose tolerance or who have risk factors for DM, a 2-h oral glucose tolerance test should be considered.
There are no recommended laboratory tests for the diagnosis of IR, and the variability among different insulin assays has made an establishment of a defined cutoff level difficult. Therefore, insulin levels should generally not be relied upon to diagnose IR. Dyslipidemia and body fat abnormalities are often accompanied by IR, and for patients with these symptoms, a 2-h oral glucose tolerance test should be considered.
Treatment Approaches
Lifestyle changes.L
Dietary guidelines established for the HIV-uninfected patient are relevant for the management of glucose disorders in the context of HIV infection [62]. Weight loss through increased activity and caloric restriction should be recommended for overweight HIV-infected patients with abnormalities in glucose metabolism.
Pharmacology.
In general, the management of glucose disorders in patients with HIV infection does not differ from that of HIV-uninfected patients; thus, relevant guidelines should be applied [62]. As in the general population, medications should be reserved for patients who have established DM. Metformin improves insulin sensitivity in patients with HIV lipodystrophy [27, 28, 40, 41] and is an effective antidiabetic medication. Because development of lactic acidosis is a rare but serious side effect, this drug should be used with caution in patients receiving an NRTI, and it is contraindicated for persons with impaired renal function.
Thiazolidinediones improve insulin sensitivity in patients with HIV lipoatrophy and are a reasonable choice for the treatment of DM in the context of HIV infection [24, 27, 28]. Weight gain and fluid retention is common with these agents, and rosiglitazone treatment may worsen hyperlipidemia [24, 29, 30].
Sulfonylureas improve plasma glucose by stimulating insulin secretion but do not reverse underlying IR. Insulin is inexpensive, well tolerated, and effective for the treatment of DM, particularly when the response to oral agents has been suboptimal. Regardless of therapeutic approach, the goal of therapy should be the normalization of glycosylated hemoglobin (A1C). In many cases, achievement of glycemic control may require combination therapy.
Antiretroviral substitution. Substitution of an NNRTI for a PI has been observed to increase insulin sensitivity in some\but not all\studies [19].
Reference:
Current Concepts in the Diagnosis and Management of Metabolic Complications of HIV Infection and Its Therapy
electronically published 27 July 2006.
Clinical Infectious Diseases 2006;43:645-653
D. A. Wohl,1 G. McComsey,2 P. Tebas,6 T. T. Brown,7 M. J. Glesby,9 D. Reeds,11 C. Shikuma,12 K. Mulligan,13 M. Dube,16 D. Wininger,5 J. Huang,15 M. Revuelta,10 J. Currier,14 S. Swindells,17 C. Fichtenbaum,4 M. Basar,18 M. Tungsiripat,3 W. Meyer,8 J. Weihe,12 and C. Wanke19
1University of North Carolina, Chapel Hill; 2Case Western Reserve University and 3Cleveland Clinic, Cleveland, 4University of Cincinnati, Cincinnati, and 5Ohio State University, Columbus, Ohio; 6University of Pennsylvania, Philadelphia; 7Johns Hopkins University and 8Quest Diagnostics\Baltimore, Baltimore, Maryland; 9Weill Medical College of Cornell University and 10Beth Israel Medical Center, New York, New York; 11Washington University, St. Louis, Missouri; 12University of Hawaii, Honolulu; 13University of California San Francisco, 14University of California, Los Angeles, and 15University of California, San Diego; 16Indiana University, Indianapolis; 17University of Nebraska, Omaha; and 18Frontier Science & Technology Research Foundation, Amherst, and 19Tufts University, Boston, Massachusetts
NRTI Use and Glucose Abnormalities
The present study conducted by Lo et al. [7] provides useful new data on the relationship between cumulative exposure to nucleoside reverse-transcriptase inhibitors (NRTIs), insulin resistance, and plasma lactate levels. In this report involving 95 HIV-infected patients (90% of whom were men, and 96% of whom were antiretroviral experienced), duration of NRTI therapy was positively correlated with lactate levels and was also associated with insulin resistance, as evaluated by the homeostatic model for assessment of insulin resistance. Other factors noted to be associated with elevated lactate levels were a decreased percentage of body fat, age, and duration of protease inhibitor therapy.
Although the present study is cross-sectional and cannot attribute causality to the observed associations, it identifies an interesting and potentially important link between lactate levels and insulin resistance. Indeed, in a multivariate analysis controlling for potential confounders, insulin resistance (as evaluated by the homeostatic model for assessment of insulin resistance) and not duration of NRTI therapy was significantly associated with lactate levels. Lo et al. [7] postulate that elevated lactate levels may directly influence insulin sensitivity, and this may be one mechanism by which NRTI exposure leads to insulin resistance. Indeed, cross-sectional studies among obese, non-HIV-infected individuals have also shown similar correlations between insulin sensitivity and baseline lactate levels [8]. Furthermore, in animal studies, direct administration of lactate led to impaired insulin-stimulated glucose uptake into muscle as a result of acute suppression of glycolysis, as well as inhibition of downstream insulin receptor substrate signaling, without any effect on glucose transporter 4 (GLUT4) [9]. On the basis of these observations and the observations made in Lo et al. [7], chronic low-grade elevations in lactate level as a consequence of NRTI exposure may contribute significantly to insulin resistance in HIV-infected patients.
The possibility remains that the effects of NRTI therapy on adipose tissue may also be important in the etiology of insulin resistance in patients with HIV infection. Furthermore, adipose-derived lactate may also be playing a role. Peripheral lipoatrophy is a recognized complication of NRTI therapy [10, 11] that may be due to mitochondrial dysfunction effecting lipid metabolism in adipocytes [12]. Several studies have demonstrated a direct association between decreased limb fat and insulin resistance in HIV-infected patients with lipodystrophy [4, 13]. Lo et al. [7] do not provide estimates of limb fat per se, but total percentage of body fat (as a surrogate marker for peripheral lipoatrophy) was inversely associated with lactate levels and duration of NRTI exposure. Increased exposure to NRTI therapy may therefore lead to fat atrophy, as well as to increased lactate levels, which, independently or in combination, may directly contribute to impaired insulin sensitivity. Of note, oxidative stress can result in decreased adiponectin expression and increased lactate production in 3T3 L1 adipocytes [14]. Mitochandrial dysfunction associated with NRTI use may create oxidative stress and subsequent increases in lactate production from adipose tissue in patients with HIV infection and thereby contribute to insulin resistance. However, adiponectin has also been identified as a factor associated with insulin sensitivity in patients with HIV infection. In vitro exposure of adipocytes to NRTIs decreases adiponectin levels and alters lipid metabolism [15], and several studies have demonstrated a strong relationship between limb fat atrophy, decreased serum adiponectin levels, and insulin resistance in patients with HIV infection [16, 17]. Oxidative stress and/or mitochondrial insult from NRTI exposure may represent a common pathway for increased lactate levels and decreased adiponectin from adipocytes, which ultimately results in insulin resistance in patients with HIV infection who are receiving antiretroviral therapy.
Of interest, in Lo et al. [7], the cumulative duration of NRTI therapy was more predictive of lactate levels than was the presence or absence of current NRTI exposure. This observation may be limited by the relatively small number of subjects who were not currently receiving an NRTI (only 6 of 95 subjects were not currently receiving an NRTI). However, if this finding is reproducible in a larger cohort, it has important implications for the long-term consequences of NRTI exposure, and future studies assessing the possible reversibility of these effects and evaluating the differential effects of thymidine analogues and NRTI-sparing regimens on lactate levels and insulin resistance are needed.
(Insulin Resistance among HIV-Infected Patients: Unraveling the Mechanism; Colleen Hadigan; Program in Nutritional Metabolism, Massachusetts General Hospital, Boston, Massachusetts; Clinical Infectious Diseases 2005;41:1341-1342).
Reyataz, Efavirenz, and Glucose
At the Lipodystrophy Workshop in Paris 2003, Joseph Jemsek (Jemsek Clinic, Huntersville, NC) reported body effects of atazanavir (Reyataz) and efavirenz each combined with fixed dose AZT and 3TC from the BMS 034 study. These are data after 48 weeks from the Metabolic Substudy of 034. So far in studies ATV has not shown elevations in cholesterol, tryglicerides, and glucose.
The purpose of the substudy is to evaluate body fat redistribution in antiretroviral-naive patients treated with the study drugs.
FASTING GLUCOSE & FASTING INSULIN
For patients taking ATV fasting glucose increased from 93 to 97 mg/dL, and fasting insulin went from 12.9 uU/mL to 12.5 uU/mL. For patients taking EFV fasting glucose went from 91 to 94 mg/dL and fasting insulin from 10 to 11.3 mg/dL.
The study authors concluded that ATV and EFV are associated with comparable effects through week 48. There was no evidence of belly central adiposity by VAT or TAT and no evience of lipoatrophy at week 48. There were no increases in lipids or insulin resistance for patients taking ATV, and patients taking EFV saw some increases in lipids. Neither ATV or EFV resulted in increases in insulin resistance.
In a randomized, double-blind, double-dummy, active-controlled, 2-arm study comparing the antiviral efficacy and safety of atazanavir 400 mg administered once daily with efavirenz 600 mg administered once daily in combination with open-label fixed-dose zidovudine plus lamivudine twice daily. The 810 treatment-naive patients were stratified by HIV RNA level. The primary efficacy end point was the proportion of treated patients with HIV RNA levels <400 copies/mL through week 48. (Comparison of Once-Daily Atazanavir With Efavirenz, Each in Combination With Fixed-Dose Zidovudine and Lamivudine, As Initial Therapy for Patients Infected With HIV; JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 36(5) 15 August 2004; Squires, Kathleen MD). Glucose and insulin levels were measured at baseline and week 48. Mean fasting glucose levels at week 48 were comparable to those at baseline for both the atazanavir and efavirenz regimens. In the atazanavir treatment group, mean fasting glucose levels were 90 mg/dL at baseline and 93 mg/dL at week 48; in the efavirenz treatment group, they were 90 mg/dL at baseline and 94 mg/dL at week 48. Through week 48, mean fasting insulin levels increased (but not significantly) in patients in the atazanavir arm (11.3 uU/mL at baseline vs. 12.3 uUmL at week 48). Among the efavirenz-treated patients, a significant (but not clinically relevant) mean increase of 1.4 uUmL in fasting insulin was observed (9.9 uUm/L at baseline vs 11.5 uUmL at week 48; p=0.04).
Taking nevirapine lowered the risk 11% per year (P = 0.0003) for diabetes in the D.A.D. Study. Taking d4T (stavudine) independently inflated the risk of diabetes mellitus in the multinational D:A:D cohort. (De Wit S, Sabin CA, Weber R, et al. 8th International Congress on Drug Therapy in HIV Infection, November 12-16, 2006, Glasgow. Abstract PL9.5.).
At ICAAC 2005, Spanish researchers (Palacios et al) reported that insulin resistance developed in 13% of ART-naive patients 48 weeks after starting HAART. HOMA >3.8 was defined as insulin resistance. 15 patients (13%) developed insulin resistance at 48 weeks of HAART. Nevirapine was associated with NOT having insulin resistance; 0 patients on NVP developed insulin resistance. 19 patients were on NVP & did not develop IR.
Our objective was to carefully characterize the virological and metabolic effects of switching from a successful protease inhibitor (PI)-based antiretroviral regimen to a nonnucleoside reverse transcriptase inhibitor (NNRTI)-based regimen with nevirapine (NVP). Forty patients, taking their first successful (less than 40 HIV RNA copies/ml) PI-based regimen, switched their PI to NVP. If patients did not tolerate NVP, substitution with efavirenz was allowed. The duration of the study was 48 weeks. At 12 weeks intervals subjects had multiple virological and metabolic parameters including glucose, insulin, C-peptide, glucagon, proinsulin, blood lipids, and lipoproteins. There were improvements in glucose (decreased fasting glucose, insulin, and improved insulin tolerance) and lipid metabolism (decreased triglycerides and increased HDL), but no changes in body composition and bone mineral density. Our study supports a pathogenic role for PIs in the development of hypertriglyceridemia and insulin resistance, but a more limited role in the fat redistribution syndrome. (AIDS Res Hum Retroviruses. 2004 Jun;20(6):589-94. * Tebas P, Washington University School of Medicine, St. Louis, Missouri 63110, USA.
At the 3rd Lipodystrophy Workshop 2001, Michael Dube, from the ACTG and Indiana University, reported on "Prospective 48-week Intensive Metabolic Study of Amprenavir-based Therapy in HIV-infected Patients". He reported finding no significant change in fasting glucose, fasting insulin. Insulin sensitivity (resistance) did not fall significantly by week 8 or week 24, but was decreased at week 48. Six patients experienced new or worsening glucose tolerance by week 24, but fasting hyperglycemia (sugar in blood) did not occur. 14 stable, non-diabetic, PI-naive adults (12 men, 2 women) with CD4 >100, HIV-RNA >500, fasting blood sugar <110 mg/dL, no OI, no meds affecting glucose metabolism received APV 1200 mg twice daily + abacavir 300 mg twice daily + 3TC 150 mg twice daily. Two patients with prior 3TC received d4T. Some patients had AIDS. They were prospectively evaluated by oral glucose tolerance test (OGTT) and IV-GTT, lipid profile measures, and DEXA at weeks 0-24-48 weeks after starting therapy.....
RESULTS
* Fasting glucose was unchanged throughout the study: 95 at baseline and 94 at week 48 (p=.97)
* Fasting insulin was 9.6 at baseline and 13 at week 48 (p=.08)
* 120-minute glucose was 114 at baseline, 139 at week 8, 146 at week 24, and 122 at week 48 (p=.35)
* Insulin sensitivity did not change from baseline (4.9) to week 8 (4.2) and week 24 (4.7), but was significantly less at week 48 (3.0)
* Free fatty acids increased at week 24 but declined to baseline levels by week 48
Dube concluded: Insulin resistance appeared late (at week 48), following and probably due to weight gain, particularly trunk fat. These findings suggest that amprenavir might be a good regimen option for patients with diabetes or a propensity for diabetes. Dube reported insulin resistance appeared late following weight gain, particularly trunk fat, but loss of limb fat or facial lipoatrophy did not occur.
|
|
| |
| |
|
|
|