| |
Utility of Standard Genotyping among ART-Naive Individuals with Unknown Duration of Infection; 25% with Drug Resistance in San Diego study
|
| |
| |
Clinical Infectious Diseases Feb 1, 2007;44:456-458
Brief Report
Davey Smith,1,2 Niousha Moini,1 Rick Pesano, 3 Edward Cachay,1 Heidi Aiem,1 Yolanda Lie,3 Douglas Richman,1,2 and Susan Little1
1University of California, San Diego, and 2Veterans Administration San Diego Healthcare System, San Diego, and 3Monogram Biosciences, South San Francisco, California
"....This study demonstrates a higher rate of HIV drug resistance among antiretroviral-naive individuals with unknown duration of HIV infection than previously described.... Twenty-six (25%) of these individuals harbored HIV strains that were resistant to at least 1 class of antiretroviral agents. Six individuals harbored HIV strains resistant to 2 classes, and 1 individual harbored an HIV strain resistant to 3 classes (table 1). K103N was the most common drug-resistance-associated mutation, appearing in sequences obtained from 12 patients....Four of the 6 patients who had the M184V mutation....most public health agencies in the United States do not routinely offer drug-resistance testing to antiretroviral-naive patients, although the prevalence of primary drug resistance has been documented at rates higher than this threshold [2, 3]. Our data suggest that routine drug-resistance testing of patients who present to a health care provider stating that they are antiretroviral naive prior to initial antiretroviral therapy would be a cost-effective clinical practice...."
Abstract: In clinical settings, we have found a high rate of human immunodeficiency virus (HIV) drug resistance among antiretroviral-naive patients for whom the duration of infection was unknown. These high rates were most likely the result of both transmitted resistance and informal antiretroviral use, and they suggest that routine resistance testing among antiretroviral-naive patients would be a cost-effective clinical practice.
Background. Research cohort studies of individuals who were recently infected with HIV in the United States, Canada, Mexico, and Europe have reported prevalence of transmitted drug resistance of 8.3%-20% [1-6]. Drug-resistance testing before initiation of antiretroviral therapy in antiretroviral-naive patients is cost-effective when the prevalence rate of transmitted drug resistance is 8%-10% [7]. Most transmitted drug resistance can be detected with standard population-based pol sequencing (genotype testing) for at least 3 years after initial infection [8-10]. However, current International AIDS Society guidelines [11] recommend drug-resistance testing for individuals whose duration of HIV infection is <1 year. Because most HIV-infected individuals do not present for medical care within the first year of their infection, some clinicians continue to question the value of drug-resistance testing to help guide antiretroviral therapy for antiretroviral-naive patients [12]. This study aimed to determine the prevalence of drug resistance among antiretroviral-naive individuals with unknown duration of infection who were receiving clinical care in public health sites in San Diego County, California, in an effort to guide clinical practice in these environments.
Methods. To address the question of whether drug-resistance testing should be routinely implemented in clinical practice, San Diego County funded a pilot program to offer standard genotypic drug-resistance testing for all antiretroviral-naive individuals who received HIV-related medical care through San Diego County public funding from 1 January 2005 to 31 December 2005. Whether to access this program was decided by each patient's clinical health care provider. Drug-resistance testing was performed and interpreted as an analysis of genotype with use of population-based sequencing of pol (Monogram Biosciences). Drug resistance was also interpreted according to the International AIDS Society 2006 guidelines [13]. Maximum likelihood phylogenetic analysis (using fastDNAmL [14]) was performed on all pol sequences to determine if viruses that harbored drug-resistance-associated mutations clustered together, thereby identifying a potential transmission network of the drug-resistant virus. Phylogenetic analysis was performed with all amino acid residues included and with those residues associated with decreased drug susceptibility removed. Clustering was considered to be significant if >3 sequences with drug-resistant mutations shared a most-common recent ancestor or if the genetic diversity between any 3 or more sequences that harbored drug-resistant mutations was <4% [15]. Patient clinical data were not routinely collected as a part of this study.
Results. Nine San Diego County HIV clinics participated in this study, and 103 individuals with an unknown duration of infection received standard drug-resistance testing (performed at Monogram Biosciences [San Francisco, CA]). Twenty-six (25%) of these individuals harbored HIV strains that were resistant to at least 1 class of antiretroviral agents. Six individuals harbored HIV strains resistant to 2 classes, and 1 individual harbored an HIV strain resistant to 3 classes (table 1). K103N was the most common drug-resistance-associated mutation, appearing in sequences obtained from 12 patients (table 2). Phylogenetic analysis of all sequences with and without amino acid residues associated with drug resistance demonstrated no significant clustering of viral sequences that would indicate a network of transmission of drug-resistant virus (data not shown).
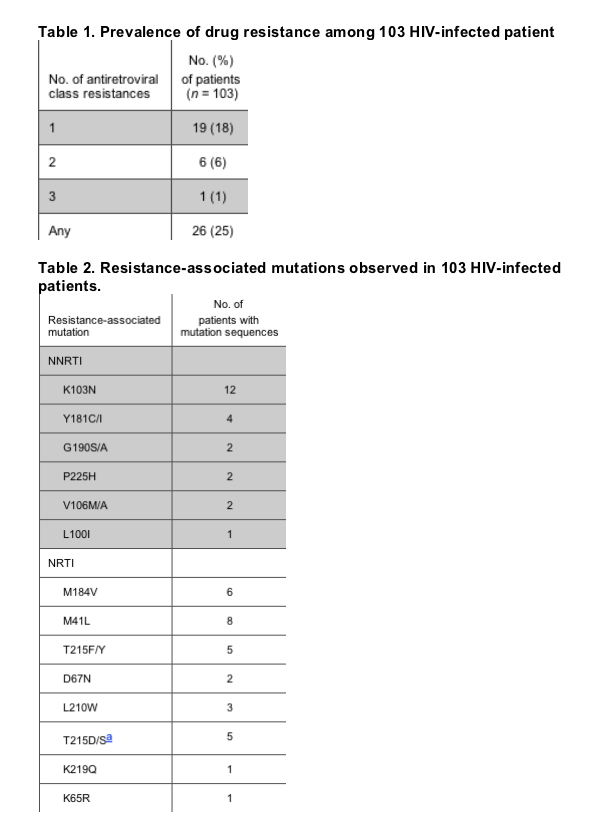
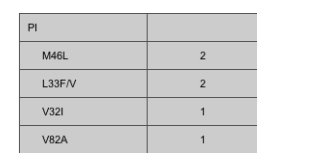
Four of the 6 patients who had the M184V mutation\which is associated with resistance to nucleoside analogue reverse-transcriptase inhibitors\also harbored drug resistance to another antiretroviral class; 3 of these patients had resistance to nonnucleoside reverse-transcriptase inhibitors and 2 had resistance to protease inhibitors as well as multiple nucleoside reverse-transcriptase inhibitor resistance-associated mutations. Two of these sequences harbored similar drug-resistance patterns and were collected from the same clinic, but were from different patients. When the clinician was questioned as to whether these 2 particular genotypes were collected under this program from antiretroviral-experienced patients by mistake, the clinician reported having questioned the patients upon receiving the results. The patients reported to their clinician that they were roommates and had both been taking a friend's HIV medications intermittently when they were feeling "poorly." Clinical data were not available for the other sequences.
Conclusion. These investigations found an extremely high prevalence of drug-resistant HIV among antiretroviral-naive patients receiving medical care in San Diego County, California, whose duration of HIV infection was unknown. This probably represents previously unrecognized transmitted and acquired drug resistance, both of which would have significant implications for the choice and effectiveness of clinician-monitored antiretroviral therapy.
This study demonstrates a higher rate of HIV drug resistance among antiretroviral-naive individuals with unknown duration of HIV infection than previously described. Our data may be different from earlier data for the following reasons: (1) our study includes a high prevalence of HIV infections among men who have sex with men (>84% of all new HIV infections in San Diego County occur among men who have sex with men [17]), which has been linked to higher rates of transmitted drug resistance [3, 18]; and (2) our patients may have engaged in the informal and undeclared use of antiretroviral medications. Regardless of the mechanism, the patients in our study presented to their clinical provider as antiretroviral naive. In addition, through this program, we performed 103 genotype tests; however, a total of 161 individuals received a new diagnosis of HIV infection at San Diego County HIV testing and counseling sites during the study period [19]. This lack of participation may indicate (1) that not all individuals who received a new diagnosis of HIV infection entered clinical care soon after the diagnosis, (2) that not all clinicians participated in the program, (3) that not all individuals who received a new diagnosis of HIV infection qualified for public funding for health care (e.g., individuals of undocumented legal status or who have veterans' benefits or private health insurance), or (4) a bias by individual clinicians to perform a genotype test only for those patients who were at the highest risk for acquiring drug-resistant HIV infection. Given these potential limitations and biases, we are unable to generalize these results to the population as a whole, despite capturing data for a significant number of patients who presented to their HIV care provider as antiretroviral naive.
In this study, we found many patients with highly drug-resistant strains of HIV, including viruses that harbored high-level drug resistance, such as M184V, that greatly reduce susceptibility to the commonly-used antiretrovirals lamuvidine and emtricitabine [20]. Although M184V is a common mutation in treated populations, it is very rare among individuals who are antiretroviral naive, probably because it reduces the replication capacity of the virus in the absence of antiretroviral pressure [21]. This led us to suspect that many of the patients whom the clinicians believed to be antiretroviral naive were, in fact, antiretroviral experienced. Although very little clinical data were available through the design of the study, we were able to document 2 instances (described above) of informal use of antiretroviral drugs. Although not evaluated in this study, another possible source of acquired drug resistance could be the administration of short-course antiretroviral therapy in the form of preexposure or postexposure prophylaxis to persons who unknowingly had a previously established HIV infection [22, 23].
Cost-effectiveness modeling has revealed that HIV drug-resistance testing is at least equivalent in cost to other HIV-related medical care practices when the regional prevalence rate of primary drug resistance is 8%-10% [3, 7]. However, most public health agencies in the United States do not routinely offer drug-resistance testing to antiretroviral-naive patients, although the prevalence of primary drug resistance has been documented at rates higher than this threshold [2, 3]. Our data suggest that routine drug-resistance testing of patients who present to a health care provider stating that they are antiretroviral naive prior to initial antiretroviral therapy would be a cost-effective clinical practice, whether the duration of HIV infection is known or unknown. In addition, we recommend ongoing patient education regarding the mechanisms of antiretroviral drug resistance and routine inquiries of patients by clinicians regarding any informal use of antiretroviral drugs.
Financial support. County of San Diego, National Institute of Allergy and Infectious Diseases (AI27670, AI043638, and AI55276), the Adult AIDS Clinical Trials Group Central Group Grant (U01AI38858), University of California, San Diego Center for AIDS Research (AI 36214), National Institutes of Health (AI29164, AI047745, and AI07384), and the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Healthcare System.
Potential conflicts of interest. R.P. and Y.L. are employees of Monogram Biosciences. D.R. is a paid consultant for Monogram Biosciences. All other authors: no conflicts.
References
1. Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347:385-94. First citation in article | PubMed | CrossRef
2. Little SJ, May S, Hecht F, et al. Increase in transmitted NNRTI drug resistance among recently HIV infected patients from North America and Australia. Antivir Ther 2006; 11:S110. First citation in article
3. Weinstock HS, Zaidi I, Heneine W, et al. The epidemiology of antiretroviral drug resistance among drug-naive HIV-1-infected persons in 10 US cities. J Infect Dis 2004; 189:2174-80. First citation in article | Full Text | PubMed
4. Brooks JI, Pilon RG, Merks HW, et al. Regional variation in HIV strain and drug resistance: the Canadian experience with a national surveillance program. Antivir Ther 2006; 11:S119. First citation in article
5. Escoto-Delgadillo M, Vazquez-Valls E, Ramirez-Rodriguez M, et al. Drug-resistance mutations in antiretroviral-naive patients with established HIV-1 infection in Mexico. HIV Med 2005; 6:403-9. First citation in article | PubMed | CrossRef
6. Wensing AM, Van De Vijver DA, Angarano G, et al. Prevalence of drug-resistant HIV-1 variants in untreated individuals in Europe: implications for clinical management. J Infect Dis 2005; 192:958-66. First citation in article | Full Text | PubMed
7. Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med 2001; 134:440-50. First citation in article | PubMed
8. Brenner BG, Routy JP, Petrella M, et al. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J Virol 2002; 76:1753-61. First citation in article | PubMed | CrossRef
9. Gandhi RT, Wurcel A, Lee H, et al. Isolated antibody to hepatitis B core antigen in human immunodeficiency virus type-1-infected individuals. Clin Infect Dis 2003; 36:1602-5. First citation in article | Full Text | PubMed
10. Barbour JD, Hecht FM, Wrin T, et al. Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 2004; 18:1683-9. First citation in article | PubMed | CrossRef
11. Hirsch MS, Brun-Vezinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1: 2003 recommendations of an International AIDS Society-USA Panel. Clin Infect Dis 2003; 37:113-28. First citation in article | Full Text | PubMed
12. Little SJ, Smith DM. HIV treatment decisions and transmitted drug resistance. Clin Infect Dis 2005; 41:233-5. First citation in article | Full Text | PubMed
13. Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: fall 2006. Top HIV Med 2006; 14:125-30. First citation in article | PubMed
14. Olsen GJ, Matsuda H, Hagstrom R, Overbeek R. fastDNAmL: a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. Comput Appl Biosci 1994; 10:41-8. First citation in article | PubMed | CrossRef
15. Hue S, Clewley JP, Cane PA, Pillay D. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS 2004; 18:719-28. First citation in article | PubMed | CrossRef
16. Violin M, Cozzi-Lepri A, Velleca R, et al. Risk of failure in patients with 215 HIV-1 revertants starting their first analog-containing highly active antiretroviral therapy. AIDS 2004; 18:227-35. First citation in article | PubMed | CrossRef
17. County of San Diego Health and Human Services Agency, HIV/AIDS Epidemiology Unit. AIDS in men who have sex with men. County of San Diego, 2004. Available at: http://www2.sdcounty.ca.gov/hhsa/documents/AIDS_in_MSM.pdf. Accessed 1 May 2006. First citation in article
18. Eshleman SH, Husnik M, Hudelson S, et al. Analysis of antiretroviral drug resistance and HIV-1 subtype among men who have sex with men recently infected with HIV-1 in the United States: the EXPLORE Study. Antivir Ther 2006; 11:S122. First citation in article
19. County of San Diego Health and Human Services Agency, HIV/AIDS Epidemiology Unit. HIV/AIDS Epidemiology Report 2005. County of San Diego, 2005. Available at: http://www2.sdcounty.ca.gov/hhsa/documents/2005AnnualReport.pdf. Accessed 20 September 2006. First citation in article
20. Gallant JE. Antiretroviral drug resistance and resistance testing. Top HIV Med 2005; 13:138-42. First citation in article | PubMed
21. Besch CL. Antiretroviral therapy in drug-naive patients infected with human immunodeficiency virus. Am J Med Sci 2004; 328:3-9. First citation in article | PubMed | CrossRef
22. Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis 2005; 41:1507-13. First citation in article | Full Text | PubMed
23. Daskalakis D, Bernstein KT, Hagerty R, et al. HIV pre- and post-exposure prophylaxis among bathhouse patrons [late breaker poster 8]. In: Program and abstracts of the 5th National STD Prevention Conference (Jacksonville, FL). Atlanta, GA: Centers for Disease Control and Prevention, 2006. First citation in article
|
|
| |
| |
|
|
|