| |
Risks of Interruptions, TIBET Study
|
| |
| |
"ART interruption guided by CD4 cell counts and plasma HIV-1 RNA levels in chronically HIV-1-infected patients"
AIDS: Volume 21(2) 11 January 2007 p 169-178
Ruiz, Lidiaa; Paredes, Rogera; Gomez, Guadalupeb; Romeu, Joana; Domingo, Perec; Perez-Alvarez, Nuriaa,b; Tambussi, Giusepped; Llibre, Josep Mariae; Martinez-Picado, Javiera; Vidal, Francescf; Fumaz, Carmina Ra; Clotet, Bonaventuraa; The TIBET Study Group
From the aFundacio IrsiCaixa and HIV Clinical Unit, Hospital Universitari Germans Trias i Pujol, Universitat Autonoma de Barcelona, Badalona, Spain and Institut Catala de Recerca Avancada (ICREA), Spain
bUniversitat Politecnica de Catalunya, Barcelona, Spain
cHospital de la Santa Creu i Sant Pau, Universitat Autonoma de Barcelona, Barcelona, Spain
dOspedale San Raffaele, Milan, Italy
eHospital de Calella, Barcelona, Spain
fHospital Joan XXIII, Universitat Rovira i Virgili, Tarragona, Spain.
Authors concluded: "In light of our findings, GTI in their current design cannot be considered as safe as continuous antiretroviral therapy. Still, this strategy may be relatively safe in patients with nadir CD4 cell counts > 350 cells/μl and pre-therapy HIV-1 RNA < 50 000 copies. More strict CD4 cell count thresholds for therapy reinitiation (e.g., 500 cells/μl) may help improve the safety of this approach while allowing some patients to reduce their exposure to therapy".
The Editorial in this journal by Ananworanich, Jintanata and Hirschel, Bernard concluded: "The SMART study was large and well executed, with results that clearly show that STI increase the risk of AIDS-defining OI and death; moreover, the hoped-for benefits in terms of decreased cardiovascular and hepatic morbidity were not seen. To put it in a nutshell, in our opinion, the results of SMART suggest: 'Once you have started HAART, you never can stop again'.
So what is the future for STI? Although quality of life is hard to quantify, many patients may feel that their 'quality of life' is improved by time off drugs, and the one in 50-100 risk of something bad happening is worth taking. Moreover, the need to interrupt therapy for drug toxicities will remain an important issue in HIV treatment. That is why efforts to make STI safer will continue, perhaps using higher CD4 cell count start and stop criteria. Given the SMART results, however, it is unlikely that another large prospective study with clinical endpoints will be funded. Preliminary data on fixed cycle STI with at least 8 weeks on HAART are encouraging, and may provide an option of a convenient STI with a 50% reduction in drug use and simplified monitoring. In the SMART study the Kaplan-Meier curves of event-free survival only diverge after 6 months, also suggesting that shorter STI might be safer".
Abstract
Objective: We evaluated the safety of CD4 cell count and plasma HIV-1 RNA (pVL)-guided treatment interruptions (GTI) and determined predictors of duration of treatment interruption.
Methods: Chronically HIV-1-infected adults with sustained CD4 cell counts > 500 cells/μl and pVL < 50 copies/ml were randomly assigned to either continue with standard antiretroviral therapy (control group, n = 101) or to interrupt therapy aimed at maintaining CD4 cell counts > 350 cells/μl and pVL < 100 000 copies/ml (GTI group, n = 100). Both groups were followed for 2 years.
Results:
There were no AIDS-defining illnesses or deaths in either group. Compared to controls, subjects interrupting therapy reduced treatment exposure by 67%, but suffered significantly more adverse events related to the intake of medication or to therapy interruption [relative hazard, 2.71; 95% confidence interval (CI), 1.64-4.49; P < 0.001), mainly due to an excess in mononucleosis-like symptoms.
While GTI subjects demonstrated improvements in the psychosocial spheres of quality of life and pain reporting, GTI had no effect on the physical aspects of quality of life.
Although both groups had a similar hazard for developing CD4 cell count < 200 cells/μl; at least 10% of subjects on GTI had CD4 cell counts < 350 cells/μl at every time point.
Drug resistance mutations were detected in 36% of subjects but were selected de novo only in subjects interrupting non-nucleoside reverse transcriptase inhibitor therapy. Lower CD4 cell count nadir, higher set-point pVL and prior exposure to suboptimal regimens were all independent predictors of the need to reinitiate treatment.
Conclusions: Overall, GTI were not as safe as continuing therapy. Despite achieving some improvements in quality of life, GTI did not reduce the overall rate of management-related adverse events.
Discussion
The main finding of our study was that the GTI strategy tested was not as safe as continuing therapy except, possibly, in patients with nadir CD4 cell counts > 350 cells/μl and pre-therapy HIV-1 RNA < 50 000 copies/ml. Although there were no AIDS-defining events, two cases of herpes zoster and one case of oral thrush developed in patients interrupting therapy, which is unsettling in light of the SMART Study results [21]. In addition, patients were more likely to have CD4 cell counts < 350 cells/μl and could develop de novo resistance to NNRTI. The addition of HIV-1 RNA safety limits to avoid persistent high-level viral replication had an impact on the causes for treatment reinitiation and may have partially contributed to the lack of AIDS clinical progression. The relatively high CD4 cell count safety threshold probably conferred additional protection against clinical progression, relative to other studies that used lower CD4 cell count thresholds to restart therapy. The downside of setting the CD4 cell count limit at 350 cells/μl was that, at each time point, there was 10% of patients with CD4 cell counts below this threshold, which may be associated with an increased risk for clinical progression [10]. In the large SMART [21] and TRIVACAN [22] trials, a GTI strategy in which antiretroviral therapy was interrupted until CD4 cell counts reached 250 cells/μl was associated with higher mortality and a higher incidence of adverse events than continuous therapy. In both studies, the absolute number of clinical complications was small and most complications were not AIDS-defining events. This may partially explain why smaller studies like ours have not, to date, detected this association.
A second finding of our study was that GTI did not decrease the overall incidence of management-related symptoms. Despite a minor reduction in antiretroviral-related adverse events, GTI were also associated with a significant increase in MNI-like symptoms following HAART withdrawal. Concerns about GTI safety when we designed this study led us to follow patients interrupting therapy more intensively. This may have partially influenced our results, leading to a higher detection of MNI-symptoms in the GTI arm than in controls. However, the incidence of MNI symptoms was similar to that seen during acute HIV infection [27]. The SMART Trial also reported an increased incidence of treatment-associated adverse effects in patients undergoing GTI [21]. The observed lack of benefit of GTI on therapy-related adverse events may further limit their clinical indications. Moreover, the improvement in quality of life in patients interrupting therapy was only observed in health areas related to the psychological and social spectrum, as well as in pain. This suggests that GTI may be more helpful in patients experiencing treatment exhaustion than in those afflicted by physical health problems.
A third important finding was the frequent detection of resistance mutations and the de novo selection of NNRTI resistance. Prior studies tended to consider that GTI did not promote resistance evolution or de novo selection of major resistant mutations, even in patients with prior experience with dual-NRTI therapy [14]. In our trial, resistance mutations were more frequently identified in patients who received mono and/or dual-NRTI therapy before HAART, although they were also detected in 16% of patients without history of suboptimal therapy. Of note, the majority of NNRTI-resistant mutations were probably selected de novo. This was probably driven by the simultaneous interruption of all drugs in patients receiving NNRTI-containing regimens. Although a simultaneous interruption strategy is not recommended, at the time this study was designed it was uncertain whether NNRTI could be simultaneously interrupted with the remaining drugs. Indeed, the optimal time for NNRTI interruption remains to be determined, and may be influenced by genetic and ethnic factors [28,29]. Therefore, our results discourage the inclusion of any drug with long plasma or intracellular half-life or low genetic barrier into GTI regimens due to the risk of de novo selection of resistance mutations.
The definition of a subgroup of patients in whom therapy can be safely interrupted has important implications for therapeutic HIV vaccine trials, which often require transient treatment interruptions after immunogenic exposure. Our study reinforces that the CD4 cell count nadir is a key predictor for the virological and immunological outcome of patients who are challenged with treatment interruptions. The only subgroup of patients with a small hazard for therapy reinitiation was precisely those with nadir CD4 cell counts > 350 cells/μl and pre-therapy HIV-1 RNA < 50 000 copies/ml. This finding is consistent with prior studies [18,19], confirming that patients with these characteristics may interrupt therapy for prolonged periods of time.
One inherent limitation of this trial is that, as a pilot study, no assumption was made to calculate the sample size and no primary endpoint was precisely defined. Additionally, due to safety concerns, patients in the GTI group received more frequent clinical monitoring than control patients.
In light of our findings, GTI in their current design cannot be considered as safe as continuous antiretroviral therapy. Still, this strategy may be relatively safe in patients with nadir CD4 cell counts > 350 cells/μl and pre-therapy HIV-1 RNA < 50 000 copies. More strict CD4 cell count thresholds for therapy reinitiation (e.g., 500 cells/μl) may help improve the safety of this approach while allowing some patients to reduce their exposure to therapy.
RESULTS
Patients
Two hundred and one patients were included into the study; 100 were randomly allocated into the GTI group and 101 into the control group. Groups were comparable at baseline (Table 1).
The most notable data in the Baseline Characteristics wes the CD4 nadir: 338-363.
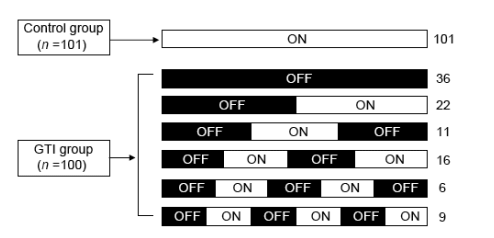
Cycles of treatment interruption and resumption in the GTI group
There was a total of 157 planned treatment discontinuations and 110 treatment resumptions in the GTI group. At week 96, 47 patients (47%) in the GTI group versus all 101 (100%) controls were receiving HAART (Fig. 1). Overall, patients in the GTI group remained without HAART for a median of more than 76 weeks [interquartile range (IQR), 36, > 92].
Fig. 1. Cycles of treatment interruption and resumption during the study follow-up. All patients in the control group received standard HAART throughout the follow-up. Among subjects interrupting therapy, 36 (36%) remained without treatment until the end of the study. The remaining patients followed serial cycles of treatment withdrawal (in black) and reinitiation (in white). Numbers at the right of the figure correspond to the number of patients belonging to each group.
The median time of exposure to antiretroviral medication was 96 (IQR, 96-96) weeks in the control group versus 28 (IQR, 0-56) weeks in the GTI group, representing a decrease in the exposure to antiretroviral treatment of 67.4% with respect to controls (P < 0.001).
The reasons for the 110 therapy resumptions were increases in plasma HIV-1 RNA load above 5.0 log10 copies/ml (42.7%), CD4 cell count decreases < 350 cells/μl (30.9%) and both virological and immunological criteria (19.1%). Two patients (1.8%) restarted therapy voluntarily and six (5.5%) due to an acute retroviral syndrome. No patient restarted HAART due to an AIDS-defining illness. Twenty-three of 30 individuals (76.7%) with nadir CD4 cell counts > 350 cells/μl and pretherapy HIV-1 RNA < 50 000 copies/ml, versus 13 of 70 (18.6%) subjects not fulfilling these criteria, were treatment-free during the 96 weeks of follow-up (P < 0.001).
Clinical progression and deaths
There were no category C AIDS-defining illnesses or deaths during the study. In the GTI arm there were two episodes of Herpes Zoster - one during treatment and another during an interruption cycle - and one episode of oral thrush during an interruption period. No category B or C AIDS-defining illnesses occurred in controls.
Adverse events and symptoms
During the 96-week follow-up, 43 patients (43.0%) in the GTI group and 16 controls (15.9%) suffered at least one adverse event related either to medication intake or to therapy cycles [relative hazard, 2.71; 95% confidence interval (CI), 1.64-4.49; P < 0.001). Forty patients of 43 in the GTI group (90.0%) experienced adverse events following antiretroviral therapy withdrawal.
The incidence of drug adverse events related to drug exposure was slightly lower in the GTI group [0.015 person-years (py) than incontrols; 0.054 py, P = 0.032]. In the GTI group, there was one case of paresthesias, one with elevation of aminotransferases and one with jaundice. Seven patients in the GTI group developed diarrhoea, six of them after interrupting therapy. Diarrhoea in the GTI group was mild and associated with other mononucleosis (MNI)-like symptoms, thus being classified as another MNI-like symptom. The most frequent drug-related adverse events in controls were polyneuropathy (four cases), diarrhoea (three cases), elevation of liver aminotransferases (two cases), pancreatitis (one case) and nephritic colic (one case).
There was an excess of mild MNI-like symptoms in the GTI arm (incidence, 0.560 versus 0.025 py in controls, P < 0.001), including asthenia, fever, adenopathy, headache, cough, chills, myalgia, diarrhoea and rash. Ninety percent of these symptoms appeared after treatment withdrawal. In addition, there were two cases of depression in each group.
BSix patients (6%) in the GTI group experienced a full-blown, ARS after the interruption of therapy. The clinical features of ARS in these patients were fever (100%), lymphadenopathy (100%), rash (66.6%), myalgia (50%), diarrhoea (50%), headache (33.3%), and pharyngitis (33.3%). Symptoms coincided with the increase on HIV-1 replication, and lasted for a median of 4 weeks after the reinstitution of antiretroviral treatment. In all cases, ARS developed during the first interruption of therapy. A similar clinical picture recurred in a second treatment interruption in only on patient.
CD4 cell counts
During the first 8 weeks after interrupting therapy, CD4 cell counts decreased a median of 289 cells/μl in the GTI group (median rate, 36.1 cells/μl per week; P < 0.001), remaining stable above 500 cells/μl thereafter (Fig. 2).
The incidence of CD4 cell count declines to 250-350, 200-250 and < 200 cells/μl was 0.185 py (37 patients), 0.050 py (10 patients) and 0.025 py (5 patients), respectively, in the GTI group; and 0.02 py (4 patients), 0 py and 0 py, respectively, in controls (P < 0.001 for all comparisons between groups). In the GTI group, the proportion of patients with CD4 cell counts < 350 cells/μl at every time-point of follow-up was always near 10% during the first year, and always 10% or slightly higher during the second year of the study. After week 44, at every time-point of follow-up there was always one patient with CD4 cell counts < 200 cells/μl, but never more than three.
As expected from the study design, at week 96 CD4 cell counts were significantly lower in patients interrupting (median, 520 cells/μl; range, 434-664 cells/μl) than in those continuing therapy (median, 789 cells/μl; range, 628-953 cells/μl) (P < 0.001). The probability of having CD4 cell counts < 350 cells/μl at week 96 was 9.1% for patients in the GTI group and 1.0% for controls (P = 0.008). The probability of having a CD4 cell count < 200 cells/μl at week 96 was 1.0% for patients under GTI and 0% for the control group (P = 0.315).
HIV-1 RNA levels
There was an early initial increase in plasma HIV-1 RNA levels reaching a peak of 4.80 (IQR, 3.71-5.45) log10 copies/ml at week 4, followed by a plateau between 4.0 and 4.5 log10 copies/ml thereafter. As expected, plasma HIV-1 RNA levels were significantly higher among patients on GTI (median, 4.33; IQR, 3.62-4.54 log10 copies/ml) than in controls (median, 1.70; IQR, 1.70-1.70 log10 copies/ml) at the end of the study (P < 0.001).
Virological failures
Only one patient in the GTI arm fulfilled the pre-specified criteria for virological failure. This subject was not able to achieve viral suppression after resuming HAART, although eventually achieved an undetectable viral load after switching to a genotype-optimized antiretroviral regimen. Nine patients had virological failure in the control group (incidence: 0.044 py), including two cases of voluntary treatment interruption, and all responded successfully to an optimized salvage regimen.
Study of the first time to reach resumption criteria and the overall time without HAART
In a univariate non-parametric survival analysis (Fig. 3), patients who were antiretroviral naive before starting HAART (log-rank test, P = 0.0120), those who were younger than 35 years (P = 0.0042), and who had a CD4 cell count nadir > 200 cells/μl (P < 0.0001) or ≥ 350 cells/μl (P < 0.0001) were more likely to remain without treatment during a longer period of time. Substantial differences between groups were found by combining CD4 cell count nadir and pre-antiretroviral therapy HIV-1 RNA levels (P < 0.0001).
Fig. 3. Kaplan-Meier plots for the time to reach HAART resumption criteria.
Overall time until reaching HAART resumption criteria in the GTI group (median plus 95% confidence interval) (upper left), and according to antiretroviral-naive status before the current HAART regimen (upper right). Age at baseline (centre left). CD4 cell count nadir lower or higher than 350 cells/μl (centre right) and a combinatory of CD4 cell count nadir (nCD4) higher or lower than 350 cells/μl plus plasma HIV-1 RNA levels (VL) greater or smaller than 50 000 copies/ml (bottom-left).
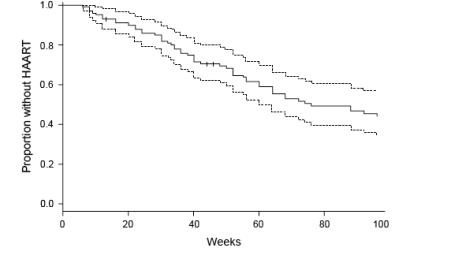
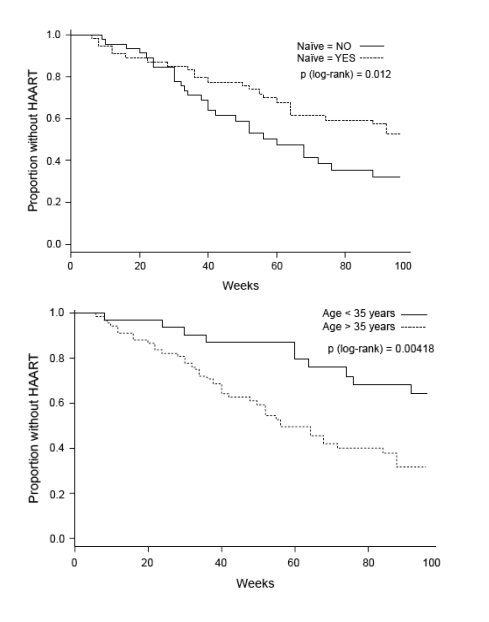
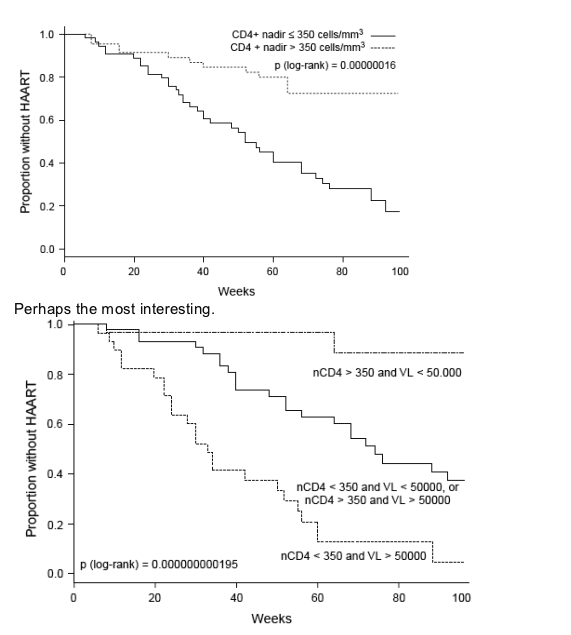
Table 2 shows factors from fitting univariate [unadjusted relative hazard (RH)] and multivariate Cox regression models (adjusted RH) related to the risk for reaching resumption criteria. In the univariate analysis, older subjects, those who had received mono or dual therapy prior to HAART, had lower CD4 cell count nadirs, and lower CD4 cell counts or higher HIV-1 RNA levels either before the initiation of any antiretroviral therapy or before the initiation of HAART, were more likely to restart therapy earlier.
In the multivariate Cox regression model, subjects with lower CD4 cell count nadirs (RH, 0.740; 95% CI, 0.670-0.905/100 cells/μl higher), higher HIV-1 RNA levels before the initiation of any antiretroviral therapy (RH, 2.271; 95%CI, 1.514-3.407, per 1 log10 copies/ml higher) and who had received a suboptimal regimen prior to HAART (RH, 0.517; 95%CI, 0.312-0.856) were more likely to resume therapy earlier. Such differences remained significant after a categorical adjustment, with subjects with CD4 cell count nadir < 350 cells/μl (RH, 2.016; 95%CI, 1.148-3.546), pre-antiretroviral therapy HIV-1 RNA levels > 5.0 logs10 copies/ml (RH, 3.759; 95%CI, 2.103-6.717), and exposure to suboptimal therapy before HAART (RH, 2.174; 95%CI, 1.284-3.676) being more likely to resume therapy.
Adherence and quality of life
No significant differences in the proportion of patients with adherence ≥ 95% were found between groups. The proportion of subjects with adherence ≥ 95% end of the study was 80% and 83% in the GTI and control groups, respectively.
Patients on GTI had significant improvements in the following quality of life dimensions at week 96: general health perception (GTI: 67.5 ± 23, Control: 46.6 ± 19; P < 0.001), pain (GTI: 84.9 ± 15, Control: 74.07 ± 26; P = 0.02), role functioning (GTI: 96 ± 12, Control: 87.8 ± 23; P = 0.05), social functioning (GTI: 97.5 ± 7, Control: 82.4 ± 21; P < 0.001) and mental health (GTI: 82.5 ± 16, Control: 70.6 ± 12; P < 0.001). Treatment interruption did not exert any discernible effect in the remaining health areas (physical functioning, energy, health problems, cognitive functioning, global quality of life and transitory health). Quality of life remained stable during follow-up among controls.
Drug resistance
Genotypic resistance was studied on a total of 87 available plasma specimens belonging to 87 patients (87%) from the GTI group. Among all treatment interruption cycles, RT-resistance mutations were detected in 31 of 87 (36%) patients and a protease-resistance mutation (M46L) was found only in one case. Twenty-six of 31 (84%) patients with RT-resistant mutations had received mono- or dual-nucleoside reverse transcriptase inhibitor (NRTI) therapy prior to HAART and 5 of 31 (16%) had no previous exposure to suboptimal therapies. The most frequent mutations were: 215Y/F/D/S (42%), 70R (39%), 67N (35%), 41L and 184V (29%), 210W and 219Q/E (19%), and 103N and 181C (13%). All RT-resistance mutations were concordant with prior drug history. Mutations associated with non-nucleoside reverse transcriptase inhibitors (NNRTI) resistance were detected in 8 of 19 (42%) patients receiving NNRTI-based regimens at the time of the study entry. These mutations were selected during treatment interruption (n = 6) or treatment resumption (n = 2) periods, except in one subject with prior NNRTI exposure in whom NNRTI-resistant mutations (103N, 181C) were already observed in proviral DNA at baseline.
In the control group, genotypic resistance analysis was assessed on plasma samples from nine patients who had virological failure or who withdrew treatment voluntarily, but only in five cases HIV-1 RNA was amplified. Drug-resistance mutations were identified in three out the five specimens and included: 41L (n = 1), 67N (n = 1), 70R (n = 2), 74V (n = 1), 103 (n = 1), 181C (n = 1), 215Y (n = 1), 219Q (n = 1).
Introduction
Despite the improvements in survival and morbidity achieved by HAART [1,2], long-term anti-HIV treatment does not permit the eradication of HIV-1 infection [3,4] and continues to be associated with substantial toxicity [5,6], adherence difficulties [7,8] and drug resistance evolution [9]. These factors may promote treatment exhaustion in some patients and pose long-term risks for the effectiveness of antiretroviral therapy. Several strategies have been proposed to reduce the antiretroviral treatment burden in patients with high CD4 cell counts, including the deferral of treatment initiation until CD4 cell counts decline below 350 cells/μl [10,11].
CD4 cell-guided treatment interruptions (GTI) have been considered potentially suitable for patients with sustained high CD4 cell counts on successful HAART who would not meet the current criteria for treatment initiation [12]. Nonetheless, the safety and clinical utility of GTI remain highly controversial issues. On one hand, cumulative data from different studies [12-20] suggest that GTI may permit prolonged treatment interruptions without major clinical complications in some well-controlled and otherwise healthy HIV-1-infected patients. On the other hand, the large SMART [21] and TRIVACAN [22] trials found that patients undergoing GTI were at significantly higher risk of severe clinical events and death than those continuing antiretroviral therapy.
If GTI could be carried out safely in certain HIV-1 infected patients, there would be essentially four reasons to undertake them: to decrease toxicity, to spare treatment options, to increase patients' quality of life, and to increase cost-effectiveness. The goal of our study was to assess the safety of GTI in chronically HIV-1 infected patients with persistently high CD4 counts and undetectable viraemia, as well as to determine predictors of prolonged treatment interruption.
Methods
Study design
This was a pilot prospective, open-label, comparative, controlled, randomized, multicentric trial conducted in chronically HIV-1-infected subjects with an adequate virological and immunological response to HAART.
Objectives
The main goals of the study were to explore the incidence of AIDS-defining illnesses and management-related adverse events, including antiretroviral-related adverse events and symptoms related to the interruption and reinitiation of treatment.
Secondary objectives were to assess the evolution of HIV-1 RNA levels and CD4 cell counts during the study, the impact of the GTI on quality of life and drug-adherence, the time until the reintroduction of therapy after GTI, the predictors of prolonged therapy interruption and the development of antiretroviral drug resistance during interruption periods.
Selection of participants and setting
The inclusion of participants began on May 2001 and finished on January 2002. The study was closed on July 15 2004. Participants were recruited in four hospitals in Spain and one hospital in Italy. This study was approved by the institutional review boards of the participating centres.
Inclusion criteria
Subjects were enrolled if they were older than 18 years old, had a Karnofky index ≥ 80, had chronic HIV-1 infection, had previously been on HAART for more than 1 year and had not interrupted their antiretroviral treatment during the prior year. Participants also had absolute CD4 cell counts ≥ 500/μl during at least 6 months and had never reached a CD4 cell nadir < 50/μl. HIV-RNA levels were < 400 copies/ml during at least 1 year and < 50 copies/ml at the study entry, as confirmed twice within 1 month. Written informed consent was obtained from patients and the study was approved by the institutional ethical committee of the public, non-profit, academic institutions of the National Health Service in Spain.
Exclusion criteria
Patients with an opportunistic infection within the prior 4 weeks, those who were pregnant or breast-feeding, and those suspected to be non-compliant a priori (as defined elsewhere [23]) were excluded from the study.
Study withdrawal criteria
The criteria for study withdrawal included the voluntary decision of the participant, the development of any AIDS-defining illness or any acute severe clinical event during the study, an improper compliance, and the suspicion or confirmation of pregnancy.
Distribution into the study arms
Participants were randomized to interrupt all drugs simultaneously or to continue the ongoing antiretroviral treatment (groups GTI and control, respectively). The randomization process was centralized in the study-coordinating centre, based on a 1: 1 random number table.
Patients allocated to the GTI group interrupted therapy until an AIDS-defining illness occurred, a severe and/or prolonged acute retroviral syndrome (ARS) took place, CD4 cell counts reached values < 350 cells/μl in two serial determinations within 1 month, or plasma HIV-1 RNA levels were ≥ 5.0 log10 copies/ml in two serial determinations within 1 month.
If any of these situations occurred, the prior HAART combination was reinitiated and was maintained until CD4 cell counts increased to > 500 cells/μl and HIV-1 RNA levels decreased to < 50 copies/ml, both in two serial determinations within 1 month. Thereafter, treatment was interrupted again. Subsequent cycles of therapy interruption and reinitiation were followed according to the previously described conditions. The control group remained on-treatment with standard HAART during the whole follow-up.
Drugs in antiretroviral combinations could be modified in both study arms at any time according to clinical and laboratory criteria. In case of virological failure associated with antiretroviral resistance, the design of the subsequent treatment line was optimized according to the corresponding genotypic analysis.
Follow-up and monitoring
Suitability for recruitment into the study was determined at two screening visits (weeks -8 and -4). Baseline time (week 0) was defined as the time of inclusion (randomization) into the TIBET study.
Thereafter, patients allocated to the control group were visited every 3 months, whereas those in the GTI group were visited every month, due to concerns about the safety of the GTI. Adverse events were pre-defined and pre-specified in the case report forms. Adherence was self-reported by the patients and calculated as the percentage of missing doses within the previous 2 weeks. Appropriate adherence was defined as the consumption of at least 95% of the medication prescribed [24]. Quality of life was assessed using the Medical Outcomes Study-HIV Health Survey (MOS-HIV) [25]. T Lymphocyte CD4 and CD8 cell counts were determined in whole blood by flow cytometry. Plasma HIV-1 RNA was quantified using the Amplicor Ultrasensitive Assay (Roche Amplicor HIV-1 Monitor assay, version 1.5) with a limit of detection of 50 copies/ml, or Amplicor HIV-1 assay, version 1.0, with a limit of detection of 400 copies/ml, following the manufacturer's instructions.
Virological failure was defined as: (a) the failure to decrease plasma HIV-1 RNA levels to less than 50 copies/ml during the first 24 weeks after the initiation or reinstitution of antiretroviral treatment, or (b) the detection of plasma HIV-1 RNA concentrations above 50 copies/ml in two consecutive determinations after achieving plasma HIV-1 RNA levels below 50 copies/ml in patients receiving HAART. Treatment interruptions violating the study protocol were also considered as failures.
Genotypic resistance testing was performed on plasma specimens from patients with confirmed virological failure in the control group and in all patients in the GTI group, 1 month after each treatment interruption. HIV-1 protease and reverse-transcriptase (RT) genes were sequenced using the TRUGENE HIV-1 Genotyping Kit and the OpenGene (version 8.0) automated DNA sequencing system, as described elsewhere [26]. Resistance mutations were reported according to the International AIDS Society-USA Drug Resistance Mutations Group report [9].
Statistical analysis
Statistical summaries of the main retrospective, baseline and prospective variables were reported. A pictorial description of CD4 cell count, HIV-1 RNA load and biochemical measurements at baseline and at times of HAART interruption and/or HAART resumption were performed. The statistical significance of the longitudinal changes in plasma HIV RNA was assessed by means of Wilcoxon, Mann-Whitney and Chi-square tests for all patients distinguishing between treatment groups.
The incidence of adverse events was reported as the number of events per person-year. A normal distribution approximation was used to compare the incidence in both groups.
Basic survival summaries were given for the first time until reaching resumption criteria ('off treatment'), and for the total time without ('off') and with ('on') treatment. Relevant covariates were chosen to compare the survival experience of the first 'time off' therapy and the overall time without HAART using Kaplan-Meier plots and Fleming-Harrington tests (including log-rank test). Whenever all the tests were significant, the log-rank test P value was reported. The significant level was set at 0.05 and all P values were two-tailed.
A Cox proportional-hazards model was used to assess the effect of baseline, pre-therapy and pre-HAART variables on the first time to reaching resumption criteria. Univariate analyses were performed using all the covariates, taking them both, when appropriate, as continuous as well as dichotomized by clinically relevant values. Cox multivariate analysis, among the statistical significant univariate covariates plus those clinically relevant, was performed (forward manner with 0.05 as the significance entry level). The final model was validated by means of the log-likelihood criteria and the Schoenfeld residuals.
Statistical analyses were performed with the use of SPSS software (SPSS, version 11.5,) and S-PLUS 2000 (version 2000 Professional Release 3) (SPSS, Inc., Chicago, Illinois, USA).
|
|
| |
| |
|
|
|