| |
Metabolic Syndrome in HIV-Infected Patients from an Urban, Midwestern US Outpatient Population
|
| |
| |
Clinical Infectious Diseases March 1, 2007;44:726-734
Kristin Mondy,1 Edgar Turner Overton,1 Jessica Grubb,1 Shao Tong,1 Warren Seyfried,1 William Powderly,2 Kevin Yarasheski1
1Washington University School of Medicine, St. Louis, Missouri, and 2University College Dublin School of Medicine and Medical Science, Dublin, Ireland
(See the editorial commentary by Jones)
This was a prospective, cross-sectional study evaluating patients' risk for the development of metabolic syndrome and cardiovascular disease. HIV-infected patients who attended the Washington University HIV Clinic (St. Louis, MO) during January-July 2005 completed a cardiovascular risk survey with their providers. Data collected were compared with data files from a cohort from the National Health and Nutrition Examination Survey (NHANES; 2001-2002) of persons who were seronegative for HIV infection who were matched for age, sex, race, and tobacco use.
".....The overall prevalence of metabolic syndrome was similar between the group HIV-infected patients and the group of persons who were seronegative for HIV infection (25.5% vs. 26.5%, respectively), .....These findings, along with a lack of independent effects of HAART on metabolic syndrome, support the notion that these traditional risk factors (i.e., hypertension, hyperglycemia, and obesity) may be more important than HAART-related factors for the prediction of metabolic syndrome and cardiovascular disease risk. ....in the HIV-infected cohort ....Despite a high prevalence of a low HDL level and high triglyceride levels, the presence of either an elevated fasting glucose level or hypertension were the strongest contributors to metabolic syndrome (OR, 11.4 and 9.3, respectively).....An important finding was the higher prevalence of dyslipidemia, despite the greater use of lipid-lowering therapy for HIV-infected patients than for HIV-uninfected persons, although these cohorts had similar prevalences of metabolic syndrome........HIV infection was a significant independent predictor of both elevated triglyceride levels and low HDL cholesterol level (P < .01 for both), after controlling for demographic and other traditional cardiac risk factors....Among HIV-related factors, although a higher CD4 cell count was an independent predictor of the development of metabolic syndrome, a higher BMI accounted for a substantial part of the CD4-attributable risk..."
The authors conclude: "although we found a high prevalence of metabolic syndrome among HIV-infected patients, this diagnosis was strongly associated with traditional cardiovascular risk factors rather than HAART-related effects. Additional longitudinal studies are needed to determine whether metabolic syndrome is equally predictive of future diabetes and heart disease in HIV-infected persons compared with HIV-uninfected persons...."
But then also say: "...an assumption has been made that HIV-infected persons with metabolic syndrome are at similar cardiovascular risk, compared with HIV-uninfected persons with metabolic syndrome, but the phenotype often differs between these 2 populations. In particular, HIV-infected persons may have significant peripheral lipoatrophy that contributes to insulin resistance and an elevated waist-to-hip ratio. Recent studies suggest that HIV-infected persons receiving HAART have a higher risk of acquiring diabetes [28] and, therefore, are likely to have a higher risk for cardiovascular disease [11]. These observations support the diagnosis of metabolic syndrome in HIV-infected persons as a likely predictor of higher risk for diabetes and cardiovascular disease, and it may serve as a simple, useful screening tool for this patient population...".
ABSTRACT
Background. The association between the use of highly active antiretroviral therapy (HAART) and an increased risk of metabolic syndrome and cardiovascular disease remains unclear.
Methods. We conducted a prospective, cross-sectional study of the risk factors associated with metabolic syndrome and cardiovascular disease among patients from an urban outpatient human immunodeficiency virus (HIV) clinic. Evaluation included laboratory data that were obtained after an overnight fast and a health survey that assessed traditional risk factors associated with cardiovascular disease, HIV-related factors, and comorbidities. Data collected were compared with data files from a cohort from the National Health and Nutrition Examination Survey (NHANES; 2001-2002) of persons who were seronegative for HIV infection who were matched for age, sex, race, and tobacco use.
Results.
Four hundred seventy-one HIV-infected subjects provided complete data. The overall prevalence of metabolic syndrome was similar between the group HIV-infected patients and the group of persons who were seronegative for HIV infection (25.5% vs. 26.5%, respectively), although the HIV-infected patients had a significantly smaller waist circumference, lower body mass index, lower high-density lipoprotein cholesterol levels, higher triglyceride levels, and lower glucose levels, compared with the subjects from the NHANES cohort. Framingham 10-year risk scores were also similar between the 2 groups.
HIV-infected patients with metabolic syndrome were more likely to be diabetic, older, and white and have a high CD4 cell count and body mass index, compared with patients without metabolic syndrome (P < .05 for all). The type or duration of antiretroviral therapy was not an independent risk factor for metabolic syndrome.
Conclusions. The prevalence of metabolic syndrome is high among HIV-infected persons, but not higher than the prevalence among HIV-uninfected persons. Traditional risk factors play a more significant role in the development of metabolic syndrome than do HIV treatment-associated factors.
Background
HAART has resulted in sustained reductions of morbidity and mortality associated with HIV infection [1, 2]. Unfortunately, HAART has also been associated with metabolic complications that may increase patients' risk of cardiovascular disease [3-5]. Specific antiretroviral therapies have been shown to increase proatherogenic lipid levels and contribute to the development of insulin resistance and visceral fat accumulation [5-7]. These characteristics are components of metabolic syndrome and are risk factors for diabetes and cardiovascular disease in the general population [8-10]. Protease inhibitors (PIs) in particular have been associated with an increased risk of cardiovascular disease [11-13], possibly in part because of their negative effect on lipids [14].
Recent studies of HIV-infected persons have revealed a high prevalence of metabolic syndrome among patients receiving HAART [15, 16]. Reported prevalence rates have been higher than rates reported for matched HIV-infected populations, yet they are similar to or even less than the 22%-24% rate of prevalence reported recently for the general US population [9]. Other data suggest that the increased prevalence of metabolic syndrome among HIV-infected persons may be more reflective of the burgeoning epidemic of obesity than a predominant effect of HAART [17]. Currently, data are lacking on risk for development of metabolic syndrome in HIV-infected persons, particularly in HIV-infected women and minorities, who represent an increasing proportion of the US and global HIV-infected population. HIV treatment guidelines recommend screening patients for metabolic complications and providing therapeutic interventions [18, 19]. Such measures may include intensified efforts to assess patients for risk factors for metabolic syndrome and cardiovascular disease as part of standard-of-care. For this study, the medical records of HIV-infected patients were reviewed to obtain risk factors for cardiovascular disease and laboratory data to (1) determine the prevalence of metabolic syndrome among a diverse HIV-infected outpatient population; (2) compare the prevalence of metabolic syndrome and diabetes with a contemporary US cohort of HIV-uninfected individuals who were matched for age, sex, race, and tobacco use; and (3) determine the risk factors for the development of metabolic syndrome that are unique to HIV-infected patients.
METHODS
Design. This was a prospective, cross-sectional evaluation of patients' risk for the development of metabolic syndrome and cardiovascular disease. As part of standard-of-care, all HIV-infected patients who attended the Washington University HIV Clinic (St. Louis, MO) during January-July 2005 completed a cardiovascular risk survey with their providers. The survey included evaluations of traditional cardiac risk factors, HIV-related factors (duration of HIV infection and HAART, current medications, and nadir CD4 cell count), comorbid conditions, and use of medications for hypertension and dyslipidemia. Providers reviewed all information with patients, verified fasting status, and obtained lipid and glucose measurements, blood pressure, height, weight, and waist circumference. Providers obtained patients' waist circumference in accordance with the National Institutes of Health (NIH) standard protocol [20]. The most recent HIV RNA level and CD4 cell count (from within 3 months of survey completion) were obtained from the medical chart and used for analysis. Pregnant women were excluded from the analysis. Traditional risk factors for metabolic syndrome and cardiovascular disease were compared with those obtained from the National Health and Nutrition Examination Survey (NHANES; 2001-2002) data files [21]. A Framingham 10-year risk score was calculated for all patients [22]. The study was reviewed and approved by the Washington University Institutional Review Board.
Definitions. According to National Cholesterol Education Program (NCEP) Adult Treatment Panel III guidelines [10], metabolic syndrome was defined as having >3 of the following criteria: abdominal obesity (waist circumference >102 cm for men and >88 cm for women), fasting triglyceride levels >150 mg/dL, high-density lipoprotein (HDL) cholesterol level <40 mg/dL for men and <50 mg/dL for women, fasting glucose levels of 100-125 mg/dL, or hypertension (blood pressure >130/85 mm Hg or current receipt of medication for hypertension). For both the NHANES cohort and the cohort of HIV-infected patients, use of a lipid-lowering agent was defined as use of a statin, fibrate, or niacin agent. An antihypertensive was any drug used to treat high blood pressure. Obesity was defined as a body mass index (BMI; calculated as the weight in kilograms divided by the square of height in meters) >30. HAART was defined as the use of 2 nucleosides (NRTIs) and a nonnucleoside reverse-transcriptase inhibitor (NNRTI), 2 NRTIs and a PI, or an NNRTI and a PI. Patients receiving triple-nucleoside therapy only were not included in this analysis.
Analysis. Data were analyzed using 2 or Fisher's exact tests for categorical variables. Continuous variables were compared using Student's t test or the Mann-Whitney U test for normally and nonnormally distributed variables. Partial correlations testing was used to test the strength of association between the diagnostic criteria for metabolic syndrome and other clinical variables for the cohort of HIV-infected patients. Significant variables were entered stepwise into a multiple logistic regression model to determine the best multivariate model. NCEP criteria for metabolic syndrome were not included in multivariate analyses. Instead, they were analyzed separately in a subanalysis focused on determining the risk (OR) of individual NCEP criterion for metabolic syndrome diagnosis. HIV RNA levels were log-transformed. All P values were 2-tailed.
For comparisons between the cohorts of patients infected with HIV and patients seronegative for HIV infection, HIV-infected patients were randomly matched to subjects from the NHANES (2001-2002) data files (Centers for Disease Control and Prevention Web site [21]). Subjects from the NHANES cohort were randomly chosen from those who matched our patients according to age (within 3 years), sex, race, and smoking status. HIV testing was only performed for subjects in the NHANES cohort who were <50 years of age; thus, we could only be certain that subjects in the NHANES cohort who were <50 years of age were not infected with HIV. However, the number of patients in the cohort of HIV-infected patients who were 50 years of age was generally low (16%), and the number of HIV-infected persons in the NHANES cohort who were 50 years of age was likely quite low. For predictors that were significantly different between the 2 cohorts, additional multiple regression analyses were performed controlling for age, race, sex, and BMI to determine predictors of individual NCEP criterion. Data were analyzed with use of the SPSS software package, version 12.0 (SPSS).
RESULTS
A total of 601 patients (394 men [66%] and 207 women [34%]) completed the metabolic survey. Sixty-one percent were African American, and 69% were currently receiving HAART; 10% were HAART naive. The proportion of women and African Americans in our clinic population was similar to that of the HIV-infected population in the United States [23]. The respective waist circumference and BMI for the entire cohort were 90.1 cm and 25.8 for men and 93.3 cm and 29.9 for women (P < .01). The mean current CD4 cell count was 449 cells/mm3. Seventy-seven percent of patients receiving HAART had an HIV RNA level <400 copies/mL.
Of the patients who completed the survey, 471 had complete laboratory data that were obtained during a period of fasting and were thus evaluated for diagnosis of metabolic syndrome (table 1). Among this subgroup, 120 patients (26%) had metabolic syndrome, and 381 patients (81%) met >1 of the criteria for risk of metabolic syndrome. During a univariate analysis that excluded the 5 NCEP criteria for metabolic syndrome, HIV-infected patients with metabolic syndrome were more likely to have a family history of cardiovascular disease or diabetes, be of white race and older age, have had a longer duration of HIV infection, and have a higher low-density lipoprotein (LDL) cholesterol level, a higher BMI, and a higher CD4 cell count, compared with HIV-infected persons without metabolic syndrome (P< .05 for all characteristics). Using multivariate analyses excluding the 5 NCEP criteria, significant predictors of metabolic syndrome among HIV-infected patients were older age, a higher CD4 cell count, white race, and a higher BMI (P < .05 for all predictors). The comparison of patients with and without metabolic syndrome revealed no significant differences with respect to the use of HAART or the type of HAART (figure 1).
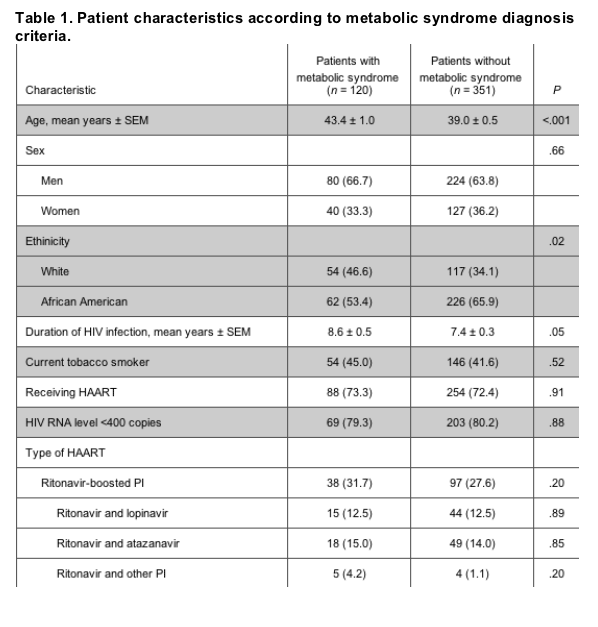
Figure 1. Type of HAART given to persons with and without metabolic syndrome. There were no significant differences between persons with or without metabolic syndrome for each of the antiretroviral therapy groups. PI, protease inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor.
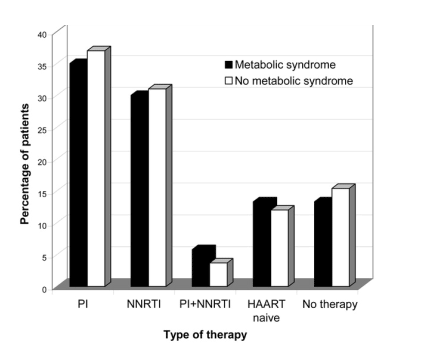
A separate analysis was performed for the patients in the HIV-infected cohort to identify the contribution of each individual NCEP criterion towards diagnosis of metabolic syndrome. Despite a high prevalence of a low HDL level and high triglyceride levels, the presence of either an elevated fasting glucose level or hypertension were the strongest contributors to metabolic syndrome (OR, 11.4 [95% CI, 6.9-18.9] and 9.3 [95% CI, 5.8-14.9], respectively).
We also explored the finding of an increased CD4 cell count as an independent predictor of the occurrence of metabolic syndrome in HIV-infected patients. An increased CD4 cell count was a strong predictor of 2 NCEP criteria (elevated triglyceride and glucose levels; P < .01) after controlling for age, sex, race, and BMI. Likewise, an increased CD4 cell count correlated with the presence of an increased number of metabolic syndrome criteria and an increased BMI (P < .01 for both; figure 2A and 2B). The change in CD4 cell count (from nadir to current value) also tended to correlate with an increased number of metabolic syndrome criteria (P = .06).
Sex and HIV-infected patients. Among HIV-infected patients with metabolic syndrome, men were significantly older, more likely to be white, have a family history of diabetes, and be receiving HAART, compared with women (table 2). Among the persons in this subgroup, men also had higher Framingham scores and triglyceride levels and lower BMI and waist circumference, compared with women (P < .01 for all).
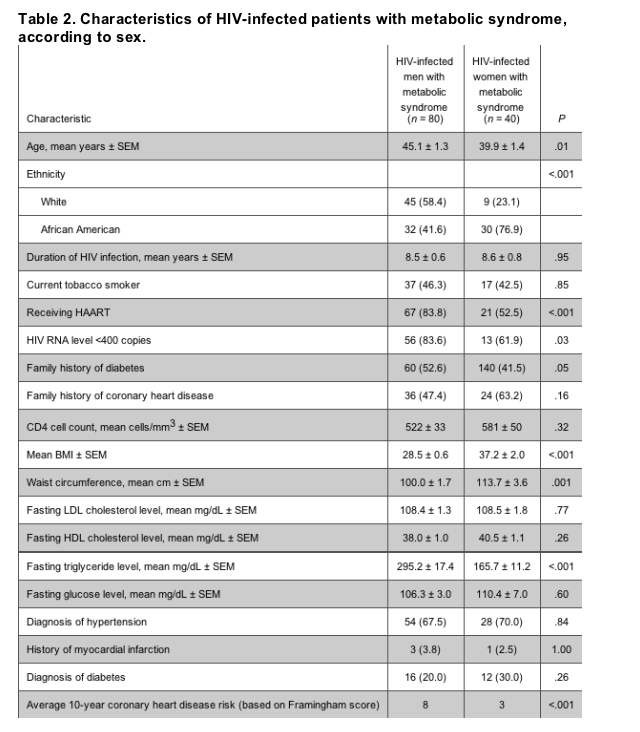
Race/ethnicity and HIV-infected patients. Among the general US population, the prevalence of metabolic syndrome appears to differ among African American and white men and women [9]; therefore, we examined this difference among the patients in the HIV-infected cohort. Among African Americans, waist circumference and BMI were significantly higher for women, compared with men. However, African American men tended to have higher levels of triglycerides and glucose and a lower HDL cholesterol level, compared with African American women. Overall, the prevalence of metabolic syndrome in the patients in the HIV-infected cohort was not significantly different between men and women when stratified by race (22% women vs. 21% men among African Americans subjects and 29% women vs. 32% men among white subjects).
The HIV-infected cohort vs. the NHANES cohort. The overall prevalence of metabolic syndrome and the Framingham scores were similar between the HIV-infected and NHANES cohorts. However, individual metabolic parameters differed significantly (table 3). HIV-infected patients with metabolic syndrome had smaller waist circumferences, lower BMIs, lower HDL cholesterol levels, higher triglyceride levels, and lower glucose levels, compared with patients in the NHANES cohort with metabolic syndrome (P < .01 for all). Despite the higher prevalence of dyslipidemia in the HIV-infected patients, compared with the patients in the NHANES cohort, nearly twice as many HIV-infected patients were already receiving lipid-lowering therapy (9.3% vs. 4.9%; P = .01).
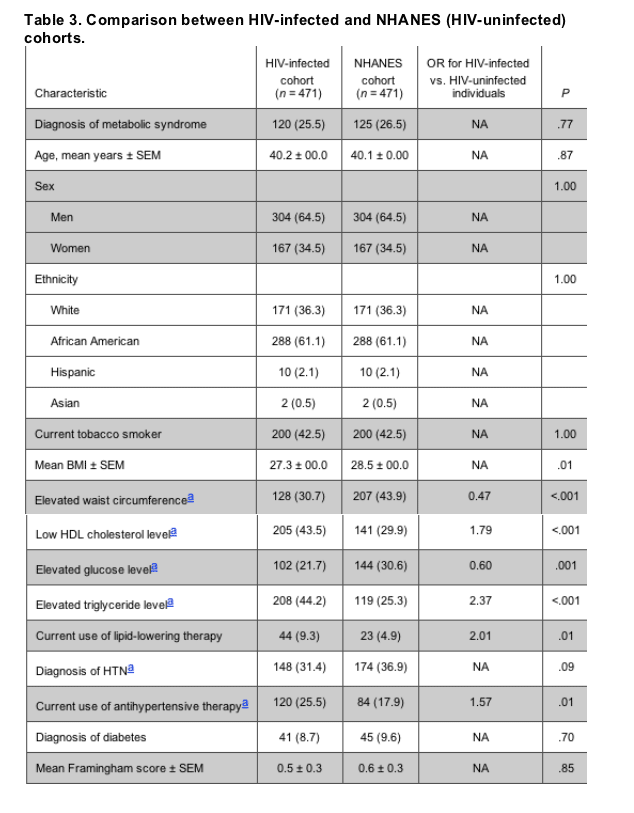
The effects of HAART. During multivariate analyses that controlled for age, sex, race, and BMI, current PI use was an independent predictor of an increased level of triglycerides only (P = .03). Patients who had a history of stavudine use were also more likely to have higher triglyceride levels than those who had no history of stavudine use (P = .05). Stavudine use was not associated with changes in BMI or waist circumference. Current NNRTI use and lower HIV RNA levels were found to be significant predictors of higher HDL cholesterol (P < .01 for both), whereas an increased duration of HIV infection was significantly associated with lower HDL cholesterol levels (P = .05). Other HIV-related predictors, such as nadir CD4 cell count, current use or duration of HAART, or current use of other specific antiretroviral drugs were not found to be significantly associated with dyslipidemia or other risk factors for metabolic syndrome during multivariate analyses. Our observations of the HIV-infected and NHANES cohorts combined revealed that HIV infection was a significant independent predictor of both elevated triglyceride levels and low HDL cholesterol level (P < .01 for both), after controlling for demographic and other traditional cardiac risk factors.
DISCUSSION
Among the patients of the urban, HIV-infected population, we found a high prevalence rate of metabolic syndrome and its associated risk factors. Nonetheless, the prevalence rate was similar to a well-matched, current population of HIV-uninfected individuals. For the patients in the HIV-infected cohort and excluding NCEP criteria, traditional factors (e.g., older age and higher BMI), higher CD4 cell count, and white race were the strongest predictors of the presence of metabolic syndrome. Unexpectedly, the duration and type of HAART were not strong predictors. Among the 5 NCEP criteria for metabolic syndrome, the presence of elevated fasting glucose or hypertension in association with HIV infection carried the highest risk of development of metabolic syndrome. Most HIV-infected patients with either of these diagnoses also tended to meet additional NCEP criteria for metabolic syndrome. In summary, among HIV-infected persons, traditional risk factors were more important predictors of metabolic syndrome than therapy-related factors.
Among HIV-related factors, although a higher CD4 cell count was an independent predictor of the development of metabolic syndrome, a higher BMI accounted for a substantial part of the CD4-attributable risk. Other studies have found a lower CD4 cell count to be associated with metabolic syndrome or increased cardiovascular risk [16, 24-26]. However, the current findings are consistent with the expected increase of weight, improved nutritional and immunologic status that occurs with effective anti-HIV therapy, and prolonged survival. The meaning of this finding is not clear, but it may reflect the physiological changes (unknown mechanisms) that accompany immune reconstitution or viral suppression. Better adherence to HAART was an unlikely factor, because similar percentages of patients with and without metabolic syndrome had an undetectable viral load. These potential links need to be examined further.
In the general US population, epidemic rates of obesity, insulin resistance, hypertension, and associated complications [9, 27] have been noted during recent years. The current findings suggest that HIV-infected persons treated with HAART are not spared from this emerging epidemic, as demonstrated by their high rates of obesity, hyperglycemia, hypertension, and dyslipidemia. These findings, along with a lack of independent effects of HAART on metabolic syndrome, support the notion that these traditional risk factors (i.e., hypertension, hyperglycemia, and obesity) may be more important than HAART-related factors for the prediction of metabolic syndrome and cardiovascular disease risk. The prevalence of diabetes was similar between the cohort of HIV-infected patients and the NHANES cohort, and mean fasting glucose levels were significantly lower in HIV-infected persons than in HIV-uninfected individuals. A recent study reported a high prevalence of diabetes in men receiving HAART [28], but these findings may also reflect the high prevalence of diabetes among US adults (estimated to be 9.6% for 2005) [29], which is comparable to the prevalence of diabetes among our clinic population. The high prevalence of hypertension among the patients in the HIV-infected cohort was also similar to the NHANES sample population and was comparable to the reported prevalence rates of hypertension for the general populations of North America and Europe [30].
An important finding was the higher prevalence of dyslipidemia, despite the greater use of lipid-lowering therapy for HIV-infected patients than for HIV-uninfected persons, although these cohorts had similar prevalences of metabolic syndrome. HIV infection is associated with an increase in triglyceride levels and a decrease in HDL level [31, 32]. Stavudine and PI use may also contribute to hypertriglyceridemia [33], which was consistent with our findings. Although dyslipidemia was predominant in the patients in our HIV-infected cohort, we found the presence of either hypertension or hyperglycemia carried a significantly more likely association with metabolic syndrome than the presence of high triglyceride levels, a low HDL level, or an elevated waist circumference. These results suggest that HIV and HAART-related dyslipidemia often occur as lone phenomena, whereas other traditional risk factors (i.e., hypertension and obesity) may occur as a cluster and, thus, carry higher risk for metabolic syndrome. Because use of lipid-lowering therapy itself was not a criterion for having metabolic syndrome, it is possible that our study underestimated the prevalence of metabolic syndrome. However, more HIV-infected patients than subjects of the NHANES cohort still had dyslipidemia that met NCEP criteria. Standard pharmacologic treatments for dyslipidemia in this HIV-infected cohort [19] were likely suboptimal because of the concurrent adverse effects of HIV infection and HAART.
Although numerous studies have implicated PIs as important risk factors for cardiovascular disease [3-7, 11-14, 24, 25], the use of PIs was not found to be an independent risk factor for metabolic syndrome when controlling for other demographic and traditional risk factors. Furthermore, when we compared the effects of specific PIs on lipid parameters and metabolic syndrome risk, we were unable to find any significant differences. However, nearly all of our patients who were receiving a PI-based regimen were using pharmacokinetic boosting with low-dose ritonavir therapy. Recent data have shown that even the "lipid-friendly" PI atazanavir may cause a small but significant elevation in triglyceride and total cholesterol levels when boosted with low-dose ritonavir therapy [34]. Although stavudine use has been associated with lipoatrophy, dyslipidemia, and insulin resistance [33, 35-37], only 24 patients who were observed at our clinic were currently receiving this drug; therefore, we likely did not have adequate power to detect any significant effect of current stavudine use on the risk for metabolic syndrome.
Surprisingly, race and sex were not independent predictors of metabolic syndrome in the patients in our HIV-infected cohort. Among African Americans in the general US population, women have a 57% higher prevalence of metabolic syndrome, compared with men [9]. Also, recent evidence indicates that the prevalence of obesity is high (30%) for African American women [17]. We found an even higher prevalence of obesity among the African American women (45%, compared with 16% of African American men) in our cohort. Among African Americans, however, men had higher levels of triglycerides and lower levels of HDL cholesterol than did women. These findings help to explain the lack of difference in the prevalence of metabolic syndrome between men and women when stratified by race. Additionally, these findings support the notion that HIV-infected men and women may have different sex-specific risk factors for the metabolic syndrome.
Our study had several limitations. First, the use of specific antiretrovirals over a long period of time may have had long-lasting, irreversible metabolic effects that were not captured in this analysis. Given the large number of patients in our cohort, it was difficult to obtain reliable, complete antiretroviral histories with regard to the duration of the use of each antiretroviral drug. Also, the use of the fasting glucose level alone to diagnose impaired glucose tolerance likely underestimated the prevalence of insulin resistance, not only among the patients of the HIV-infected cohort, but also among the subjects of the NHANES cohort. Currently, oral glucose tolerance testing is not routine, standard-of-care for HIV infection and can be difficult to obtain, even from patients at risk for diabetes. Finally, an assumption has been made that HIV-infected persons with metabolic syndrome are at similar cardiovascular risk, compared with HIV-uninfected persons with metabolic syndrome, but the phenotype often differs between these 2 populations. In particular, HIV-infected persons may have significant peripheral lipoatrophy that contributes to insulin resistance and an elevated waist-to-hip ratio. Recent studies suggest that HIV-infected persons receiving HAART have a higher risk of acquiring diabetes [28] and, therefore, are likely to have a higher risk for cardiovascular disease [11]. These observations support the diagnosis of metabolic syndrome in HIV-infected persons as a likely predictor of higher risk for diabetes and cardiovascular disease, and it may serve as a simple, useful screening tool for this patient population.
In conclusion, although we found a high prevalence of metabolic syndrome among HIV-infected patients, this diagnosis was strongly associated with traditional cardiovascular risk factors rather than HAART-related effects. Additional longitudinal studies are needed to determine whether metabolic syndrome is equally predictive of future diabetes and heart disease in HIV-infected persons compared with HIV-uninfected persons.
|
|
| |
| |
|
|
|