| |
Growth Hormone-Releasing Hormone in HIV-Infected Men With Lipodystrophy
|
| |
| |
A Randomized Controlled Trial
JAMA. July 2004;292:210-218.
Polyxeni Koutkia, MD, MA; Bridget Canavan, BA; Jeff Breu, BS; Martin Torriani, MD; John Kissko, MS; Steven Grinspoon, MD
Massachusetts General Hospital Program in Nutritional Metabolism and Neuroendocrine Unit (Drs Koutkia and Grinspoon, Ms Canavan) and Department of Radiology (Dr Torriani and Mr Kissko), Harvard Medical School, Boston, Mass; General Clinical Research Center, Massachusetts Institute of Technology, Cambridge (Mr Breu).
"....use of high-dose growth hormone (GH) may be associated with insulin resistance and fluid retention, myalgias, and other adverse effects.... Although restoration of normal GH levels may be possible and safe with low-dose GH administration, another approach is to treat with a natural GH secretagogue, such as growth hormone-releasing hormone (GHRH), to restore a more physiologic pattern of GH than that achieved with pharmacologic GH therapy.... With GHRH, feedback inhibition of insulin-like growth factor 1 (IGF-1) (a hepatic factor the secretion of which is stimulated by GH and which is responsible for many of its actions) may help to prevent excessive GH secretion and adverse effects..... exogenous GH administration is not significantly affected by feedback inhibition from IGF-1, titration is more difficult, and adverse effects may occur relating to GH excess.... Prior studies indicate that levels of IGF-1 are lower by approximately 100 ng/mL in HIV-infected patients with lipodystrophy,13 and the GHRH regimen used in this study increased IGF-1 to this degree..... Growth hormone-releasing hormone is generally well tolerated and adverse effects have been shown to occur in a minority of patients..... With excess dosing, adverse effects similar to those observed with high-dose GH may occur, including arthralgias and fluid retention.... In this study: Levels of fasting glucose, HbA1c, fasting insulin, total cholesterol, and triglycerides, as well as glucose area under the curve, insulin area under the curve, and lipid profile, did not change significantly between treatment groups (Table 2)..."
ABSTRACT
Context Reduced growth hormone (GH) concentrations are observed in men with human immunodeficiency virus (HIV) lipodystrophy.
Objective To investigate the effects of growth hormone-releasing hormone (GHRH), a GH secretagogue, in treatment of HIV lipodystrophy.
Design, Setting, and Participants Randomized, double-blind, placebo-controlled trial conducted at a research center in the United States between October 2002 and June 2003 and enrolling 31 HIV-infected men aged 18 to 60 years with evidence of lipodystrophy.
Interventions Participants were assigned to receive GHRH (1 mg subcutaneously twice daily) or placebo for 12 weeks.
Main Outcome Measures The primary outcome was change in concentrations of insulin-like growth factor 1 (IGF-1) to detect overall change in GH levels in response to GHRH. Secondary end points included body composition by dual-energy x-ray absorptiometry and computed tomography, lipodystrophy ratings, and levels of glucose, insulin, and lipids.
Results
Mean (SD) IGF-1 concentrations increased significantly in the GHRH group vs the placebo group (104 [110] ng/mL vs 6 [44] ng/mL, P = .004).
Lean body mass significantly increased in the GHRH group vs the placebo group (0.9 [1.3] kg vs -0.3 [1.7] kg, P = .04), trunk fat significantly decreased (-0.4 [0.7] kg vs 0.2 [0.6] kg, P = .03), and the ratio of trunk to lower extremity fat improved significantly (-0.22 [0.32] vs 0.14 [0.29], P = .005).
Abdominal visceral fat was reduced (-19.2 [36.6] cm2 vs 2.3 [24.3] cm2, P = .07) and the ratio of abdominal visceral fat to abdominal subcutaneous fat improved significantly more in the GHRH group (-0.19 [0.28] vs 0.07 [0.27], P = .02).
Both physician and patient rating of lipodystrophy in the arms, legs, and abdomen also improved significantly.
Levels of glucose, insulin, and lipids did not change significantly.
Conclusions GHRH was well tolerated and effectively increased levels of IGF-1 in HIV-infected men with lipodystrophy. Total and regional body composition improved in response to GHRH, with increased lean mass and reduced truncal and visceral fat. Use of GHRH may potentially be a beneficial treatment strategy for this population.
INTRODUCTION
The human immunodeficiency virus (HIV) lipodystrophy syndrome is highly prevalent among patients receiving antiretroviral therapy.1 Although heterogeneous in its presentation, the syndrome is often characterized by excessive truncal and visceral adiposity, subcutaneous and extremity fat loss,2 and metabolic abnormalities including hypertriglyceridemia, reduced high-density lipoprotein cholesterol levels, and insulin resistance, which may increase risk of coronary artery disease.3-4 Cross-sectional studies do not uniformly show an association between lipodystrophy and antiretroviral therapy,5 but longitudinal cohort studies suggest that protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs) may be associated with loss of peripheral fat.6 Prospective studies of body composition in antiretroviral-naive individuals suggest loss of extremity fat and gain in truncal fat with institution of highly active antiretroviral therapy.7 In a randomized study of antiretroviral-naive individuals initiating antiretroviral therapy, use of a stavudine-containing regimen was associated with a greater loss of limb fat than was use of other NRTIs, and use of nelfinavir was associated with greater loss of extremity fat than an efavirenz-based regimen.8 It is unknown whether newer, low-dose, "boosted" PIs are less significantly associated with the development of lipodystrophy. Accumulation of truncal fat is a significant cardiovascular risk factor in non-HIV-infected patients,9-10 and preliminary data suggest that the severity of the metabolic abnormalities increases in association with excess visceral adiposity in patients with HIV.11 Men with HIV lipodystrophy have reduced levels of growth hormone (GH) secretion in association with excess visceral fat,12-13 and this may further contribute to increased cardiovascular risk in this population.
To date, no effective therapy has been established to treat the lipodystrophy syndrome. Use of GH is a potentially appealing strategy to treat the lipodystrophy syndrome because of its known lipolytic actions to reduce visceral fat in GH-deficient patients14 and because of the recent data suggesting low GH concentrations in individuals with HIV lipodystrophy.12 However, use of high-dose GH may be associated with insulin resistance and fluid retention, myalgias, and other adverse effects.15-19 Although restoration of normal GH levels may be possible and safe with low-dose GH administration, another approach is to treat with a natural GH secretagogue, such as growth hormone-releasing hormone (GHRH), to restore a more physiologic pattern of GH than that achieved with pharmacologic GH therapy. With GHRH, feedback inhibition of insulin-like growth factor 1 (IGF-1) (a hepatic factor the secretion of which is stimulated by GH and which is responsible for many of its actions) may help to prevent excessive GH secretion and adverse effects.20-21 In contrast, exogenous GH administration is not significantly affected by feedback inhibition from IGF-1, titration is more difficult, and adverse effects may occur relating to GH excess. We used concentration of IGF-1 as the primary end point of the study to assess the changes in the GH axis in response to GHRH. Because GH is pulsatile, use of IGF-1, an integrated measure of GH secretion, is better suited to assess increases in GH secretion. Prior studies indicate that levels of IGF-1 are lower by approximately 100 ng/mL in HIV-infected patients with lipodystrophy,13 and the GHRH regimen used in this study increased IGF-1 to this degree.
RESULTS
Participant Characteristics
The 12-week treatment phase of the study took place from October 9, 2002, through June 15, 2003. Two of the 31 participants were unable to complete the protocol (Figure 1). One participant, assigned to the placebo group, withdrew from the study immediately after the baseline visit because of poor vision and an inability to learn the injection techniques. A second participant, assigned to the treatment group, was incarcerated after the baseline visit and unavailable to continue with the study. Two additional participants in the placebo group discontinued the study medication after the baseline visit because of adverse events (chest pain and rash) but agreed to remain in the study (without receiving further doses of study drug) and return for all subsequent study evaluations.
Clinical and demographic characteristics for the HIV-infected participants are shown in Table 1. Baseline characteristics, including age, weight, racial demographics, and risk factors for HIV infection, did not differ between the treatment groups (P>.05 for all baseline comparisons) (Table 1). [Mean age was 45; 70% white; duration of HIV: 12 yrs; 90% MSM]. The percentages of men using PI-, NRTI-, and NNRTI-containing regimens did not differ between the groups (Table 1). At baseline, use of individual drugs among the study participants was: abacavir (16%), abacavir in combination with lamivudine and zidovudine (Trizivir; GlaxoSmithKline, Research Triangle Park, NC) (10%), amprenavir (3%), didanosine (19%), efavirenz (39%), indinavir (3%), lamivudine (32%), lopinavir in combination with ritonavir (Kaletra; Abbott Laboratories, Abbott Park, Ill) (16%), nelfinavir (10%), nevirapine (23%), ritonavir (10%), saquinavir (3%), stavudine (39%), tenofovir (23%), zidovudine (6%), and zidovudine in combination with lamivudine (Combivir; GlaxoSmithKline) (16%). The rate of stavudine use was not statistically different (20% vs 56% for GHRH vs placebo, P = .07). Also, controlling for stavudine use did not affect the results for trunk and extremity fat (Grinspoon et al, unpublished data, May 2004). There were no significant differences for any of the baseline antiretroviral drugs except for nevirapine (40% vs 6% for GHRH vs placebo, P = .04). However, nevirapine may not have an effect on body composition.38-39 Moderate to severe fat accumulation in the abdomen was observed in 94% of the men, moderate to severe fat loss in the legs or arms in 87%, and moderate to severe fat loss in the face in 84%, as determined by physician rating of lipodystrophy at baseline.
Changes in response to GHRH treatment for metabolic and body composition variables are shown in Table 2. Variables did not differ at baseline between treatment groups (P>.05 for all baseline comparisons). Mean (SD) concentrations of IGF-1 increased significantly in the GHRH group compared with the placebo group in response to GHRH (104 [110] ng/mL vs 6 [44] ng/mL for GHRH vs placebo, P = .004). Lean body mass increased significantly (0.9 [1.3] kg vs -0.3 [1.7] kg, P = .04), whereas total fat mass did not change significantly between the groups (0.0 [1.3] kg vs -0.1 [1.4] kg, P = .80). By DXA, lower extremity fat increased in the GHRH group vs the placebo group (0.2 [0.4] kg vs -0.1 [0.3] kg, P = .05), whereas trunk fat decreased significantly (-0.4 [0.7] kg vs 0.2 [0.6] kg, P = .03), with a significant change in the ratio of trunk fat to lower extremity fat (-0.22 [0.32] vs 0.14 [0.29], P = .005) (Table 2).
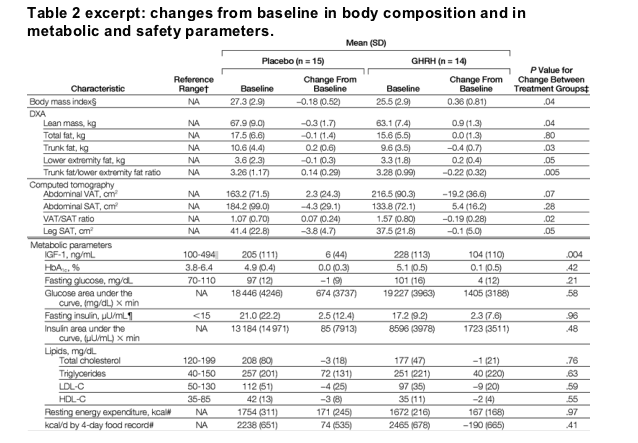
By CT, abdominal VAT decreased on average 9% in the GHRH group and increased 1% in the placebo group (-19.2 [36.6] cm2 vs 2.3 [24.3] cm2 for GHRH vs placebo, P = .07) (Table 2). In contrast, abdominal SAT increased 4% in the GHRH-treated group and decreased 2% in the placebo group, although these changes were not significant. The net overall effect of GHRH on the ratio of abdominal VAT to abdominal SAT was significant and amounted to a 12% decrease in the GHRH group vs a 7% gain in the placebo group (P = .02) (Table 2). Subcutaneous fat at the mid thigh decreased less in the GHRH group vs the placebo group (-0.1 [5.0] cm2 vs -3.8 [4.7] cm2, P = .05).
Levels of fasting glucose, HbA1c, fasting insulin, total cholesterol, and triglycerides, as well as glucose area under the curve, insulin area under the curve, and lipid profile, did not change significantly between treatment groups (Table 2). One person in the GHRH group developed an asymptomatic fasting glucose level greater than 126 mg/dL (7.0 mmol/L) at the end-of-study visit (P = .22). Neither CD4 cell count nor viral load changed significantly between treatment groups (Table 2).
Physician and patient rating of lipodystrophy improved significantly in the arms, legs, and abdomen in the GHRH group vs the placebo group (Table 3). Participants were also asked to answer an exit question at the final visit as to how their overall appearance compared with that 3 months previous. The percentages of men answering were as follows: much worse (0% vs 0% for GHRH vs placebo), somewhat worse (0% vs 20%), no difference (21% vs 53%), somewhat better (50% vs 27%), much better (29% vs 0%) (overall P = .005 by likelihood ratio). Initial lipodystrophy ratings did not correlate with change in overall appearance as assessed by exit questionnaire.
Pulse dynamics were determined from every 20-minute frequent sampling. The number of secretion peaks did not change between the 2 groups, but the peak height decreased whereas the valley mean level and nadir increased significantly in the GHRH group vs the placebo group (Table 4).
Compliance with study drug was assessed as described above and was 94% for the GHRH group and 95% for the placebo group (P = .86). One person in each group discontinued antiretroviral medications. Two men in the GHRH group changed therapy: 1 switched from an NRTI/NNRTI regimen to an NRTI/PI regimen and the second added another NRTI. Use of PIs, NRTIs, and NNRTIs were not different between the groups at the end-of-study visits (end-of-study PI use: 29% vs 40% for GHRH vs placebo, P = .52; NRTI use: 79% vs 87%, P = .56; NNRTI use: 64% vs 53%, P = .55). Use of individual drugs did not change significantly between groups over the course of the study (Grinspoon et al, unpublished data, February 2004).
Blood pressure did not increase in the GHRH group (Table 2), and no participant experienced edema, arthralgias, or any other symptoms of GH excess. No participant in the GHRH group dropped out of the study due to adverse effects. Three months after completion of the study, 1 participant in the GHRH group with a history of anal warts was diagnosed with anal carcinoma. The participant's IGF-1 level did not exceed the normal range during the study. This event was believed by the data and safety monitoring board not to be related to GHRH.
The study was powered for the primary end point only. Although many secondary end points changed significantly in response to GHRH, the study may have been underpowered to detect changes in certain variables, eg, abdominal VAT, which approached statistical significance. Post hoc power analysis showed that the study was well powered to detect significant changes in the primary safety parameters, eg, levels of glucose and HbA1c (see "Statistical Methods" section).
COMMENT
In this study, we investigated the novel use of GHRH to restore physiologic GH levels in HIV-infected men with lipodystrophy. Restoration of physiologic GH levels has been investigated in non-HIV-infected patients with abdominal obesity,40 but prior studies have only used administration of pharmacological GH and not previously investigated GHRH in the HIV population. Body composition improved without adverse effects on glucose levels or GH excess, suggesting the potential usefulness of this strategy in the HIV population.
In the HIV population, markedly reduced mean GH secretion and pulse area are observed in association with increased visceral adiposity.13 Reduced GH secretion is observed in other populations with abdominal obesity41 and may further contribute to increased abdominal adiposity, in a vicious cycle of adiposity and reduced GH. Among non-HIV-infected individuals, restoration of more normal GH concentrations results in improved fat distribution and improved metabolic parameters.40 Our data suggest that interruption of the GH-adiposity cycle by restoration of more normal GH concentrations can result in an improved pattern of fat distribution. The study was approved for men by the institutional review board and the General Clinical Research Center Scientific Advisory Committee because of the known effects of sex on the GH axis.42-45 Seventy-one percent of the population was white, and further studies in women and minorities will be important.
A number of prior studies have investigated GH administration in HIV-infected patients and shown positive effects on abdominal fat, but all such studies have used administration of pharmacological GH. For example, Wanke et al15 investigated a GH dose of 6 mg/d in an open-label study and demonstrated a reduction in abdominal adiposity, but glucose level increased significantly in 1 patient and complaints of myalgias and stiffness were common. Engelson et al18 demonstrated an approximate 50% reduction in abdominal VAT after 24 weeks in response to growth hormone at 6 mg/d in an open-label study, but 3 participants developed diabetes. Lo et al19 used a lower dose of 3 mg/d in an open-label study of 8 patients, and observed beneficial effects on trunk fat, but IGF-1 levels increased to 3 times the upper end of the normal range, and glucose intolerance worsened initially in all patients. Although glucose levels tended to return to normal, 1 patient developed overt diabetes mellitus and arthralgias necessitating dose reduction, and 2-hour glucose levels as well as glucose area under the curve remained increased at the end of the study compared with baseline.19 Kotler et al46 reported on the results of a large, double-blind, randomized, dose-finding study of GH in individuals with HIV and with lipodystrophy and marked visceral obesity, demonstrating significant reductions in visceral fat in the 4-mg daily group, but not in the group receiving 4 mg on alternate days. Significant reductions in trunk fat determined by DXA, ratio of trunk fat to limb fat, and levels of serum total and non-high-density lipoprotein cholesterol were observed at both dosages. Insulin resistance tended to worsen initially but then improve toward baseline with continued therapy.
Endogenous GHRH is a natural secretagogue for GH.47 Prior studies have used GHRH to restore normal GH levels and increase levels of IGF-1.22 Once-daily dosing with nightly GHRH is less effective than multiple daily doses of GHRH,48 and we chose twice-daily dosing.
The use of GHRH to restore physiologic GH levels is less often associated with adverse effects than is GH itself and may reduce abdominal adiposity without inducing insulin resistance, fluid retention, or myalgias,35 because IGF-1 feedback inhibition remains intact. Corpas et al22 investigated the use of GHRH (Geref; 1 mg subcutaneously twice per day) in elderly men to restore levels of IGF-1 and physiologic GH without an increase in glucose levels. No adverse effects were reported. A longer-term study using GHRH in children also demonstrated excellent tolerability and efficacy.35
Our data demonstrate that physiological increases in GH levels after administration of GHRH result in fat redistribution that includes an improved ratio of abdominal VAT to SAT. Similar changes in fat distribution with sparing of subcutaneous fat have been shown in response to low-dose GH in non-HIV-infected GH-deficient patients.14, 49 Although further studies will be necessary to determine the long-term effects of GHRH on body composition, our data suggest that GHRH at the dose and duration used in this study is not lipolytic for subcutaneous fat.
In this study, we did not observe adverse effects on levels of glucose and HbA1c. It is unlikely that we would have detected clinically significant changes, even with a much larger population based on a post hoc power analysis. Individuals with diabetes mellitus were excluded from this study, and larger increases in glucose levels may be observed in those with higher baseline levels. No significant changes in levels of cholesterol or triglycerides were observed between treatment groups, but the study may have been underpowered to detect such changes based on the variability in responses. GHRH was well tolerated. Blood pressure did not differ between groups, and no evidence of edema or GH excess was observed in participants receiving GHRH. Further studies of longer duration will be needed to confirm the absence of clinically significant effects of GHRH on glucose levels in HIV-infected patients, and those with significant elevations in blood glucose levels should be excluded from such studies. In addition, individuals with underlying malignancy are not appropriate for treatment with GHRH. Further long-term studies are needed to determine if changes in fat distribution from administration of GHRH are associated with improvement in insulin resistance and to determine the durability and optimal dosing duration for GHRH.
Immunological parameters remained stable. Growth hormone has been shown to increase CD4 cell counts and thymic mass in patients with HIV.50 In addition, GH secretagogues have been shown to have immunomodulatory effects in both animal51 and human52 studies. Further studies are needed to determine the long-term effects of GHRH on immune function in HIV-infected patients.
Our data suggest a mixed effect on pulse dynamics, with increased nadir and valley but decreased peak height in response to GHRH vs placebo. One potential explanation of our data is a direct stimulatory effect of GHRH to increase the basal GH secretion and nadir, with feedback inhibition of increased IGF-1 on peak height.
No established therapy yet exists to treat patients with HIV and lipodystrophy. The novel strategy investigated in this study, treatment with GHRH, addresses a physiologic abnormality, eg, reduced GH levels, to achieve physiological increases in GH levels and significant changes in fat redistribution. GHRH is not currently approved for chronic treatment of HIV lipodystrophy and not commercially available, approved, or recommended for any indication in adults. The cost of such a treatment is unknown. However, an estimate based on the previously published price in children53 would be approximately $1775 per month at 2 mg/d. Twice-daily subcutaneous injections may not be feasible, but other GH secretagogues dosed orally or by less-frequent injections may be developed and useful to study in the HIV population. Treatment of reduced GH levels might alternatively use low-dose physiologic GH to reduce visceral fat and improve ratios of central fat to peripheral fat. Metformin54-55 and rosiglitazone56-58 may improve insulin sensitivity, and rosiglitazone may increase subcutaneous fat.56, 58 It is unknown how long a patient would need to continue to receive treatment with GHRH.
The present study provides initial evidence of the principle that restoration of physiologic GH levels may be beneficial in HIV-infected men with reduced GH levels and suggests the need for further studies using GHRH, other secretagogues, or physiologic GH to improve fat distribution in HIV-infected patients.
METHODS
Patients
From 2002 to 2003 (individuals were screened from September 10, 2002, through January 31, 2003), 31 HIV-infected men with HIV-related lipodystrophy were recruited through community advertisements and contact with physicians in the multidisciplinary HIV practice at the Massachusetts General Hospital in Boston. The lipodystrophy was not congenital and was acquired in all participants in the context of treatment of HIV disease. Participants were asked to report when they first noted fat redistribution, and the mean (SD) duration of self-reported lipodystrophy was 32 (22) months.
Inclusion criteria included: men aged 18 to 60 years previously diagnosed with HIV infection; stable antiviral regimen for at least 6 weeks prior to enrollment; waist-to-hip ratio of 0.90 or greater; and evidence of at least 1 of the following recent changes: (1) increased abdominal girth, (2) relative loss of fat in the extremities, and (3) relative loss of fat in the face. A similar algorithm was previously used to identify patients with HIV having fat redistribution and decreased levels of GH.12 Participants were excluded if they had diabetes mellitus (defined as fasting blood glucose >126 mg/dL [7.0 mmol/L]); body mass index less than 20; hemoglobin concentration less than 9 g/dL; or if they had used GH, GHRH, oral or parenteral glucocorticoids, megesterol acetate, or antidiabetic agents within the 3 months prior to study initiation.
The protocol was approved by the institutional review board of the Massachusetts General Hospital and written informed consent was obtained from each participant prior to testing (ie, at the beginning of the study, before any tests were performed), in accordance with the Subcommittee on Human Studies at the Massachusetts General Hospital.
The primary outcome was change in levels of IGF-1 to detect overall changes in levels of GH in response to GHRH. Secondary end points included body composition determined by dual-energy x-ray absorptiometry (DXA) and computed tomography (CT); lipodystrophy ratings; levels of glucose, insulin, lipids, and glycosylated hemoglobin (HbA1c); energy expenditure; caloric intake; blood pressure; CD4 cell count; viral load; and GH pulse dynamics.
Clinical Research Protocol
After a 12-hour overnight fast, participants reported to the General Clinical Research Center (GCRC) at the Massachusetts General Hospital for a screening visit for measurement of glucose levels and complete blood cell count. Data regarding past and current use of antiretroviral medications were provided by the participant during the medical history intake and recorded. Participants had a physical examination that included measurement of the neck, mid arm, trunk, and waist-to-hip ratio. Eligible participants returned to the GCRC for an inpatient baseline visit, outpatient safety visit at 1 and 6 weeks, and an inpatient end-of-study visit at 12 weeks.
During the baseline and end-of-study visits the following data were collected after a 12-hour overnight fast: (1) height and weight; (2) levels of IGF-1, glucose, HbA1c, insulin, cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides; (3) 75-g oral glucose tolerance test to determine levels of glucose and insulin; (4) CD4 cell count and viral load; (5) resting energy expenditure and 4-day food record; (6) whole-body DXA scan; (7) single-slice abdominal CT scan at L4 and mid thigh; (8) physician and participant rating of lipodystrophy; and (9) overnight GH sampling, from 7 PM to 7:40 AM every 20 minutes.
After baseline testing was completed, participants were randomly assigned to receive daily subcutaneous injections of Geref (GHRH 1-29; Serono, Rockland, Mass) (1 mg every 12 hours) or identical placebo. An investigational new drug application was filed for the use of GHRH in this study, but an exemption was granted by the US Food and Drug Administration. The GHRH dose was determined from a prior study in elderly persons22 in which a similar dose of GHRH (1 mg subcutaneously twice daily) was safely used in elderly men to restore physiologic GH levels over 14 days.22 Randomization codes were available to the study statistician and the Massachusetts General Hospital pharmacy, but not to study investigators. Placebo was manufactured by the Massachusetts General Hospital pharmacy and was identical to active drug in color, consistency, and packaging. Participants received instruction on self-administration of the study medication by the nursing staff of the GCRC and study drug administration was witnessed at the baseline and 1-week visits to ensure proper technique. Compliance history and vial count were performed at each visit. Compliance was measured by return of used vials and review of medication use at each subsequent visit by the investigator and nursing staff. Missing or broken vials were assumed to be unused. Of those men completing the study (n = 29), 2 were unable to continue with study drug injections; for those 2 men and for 2 men (1 in each group) who were lost to follow-up, compliance was not calculated.
Body Composition Analysis
Whole-body and regional fat was determined by DXA. The technique has a precision error, in our laboratory, of 1.7% for fat and 2.4% for fat-free mass. Lower extremity fat, trunk fat, and trunk-to-extremity ratios were assessed using DXA as in prior studies.23 Single-slice cross-sectional abdominal CT scanning was also performed to assess the relative distribution of abdominal subcutaneous adipose tissue (SAT), abdominal visceral adipose tissue (VAT), and mid-thigh SAT as previously described.24
Laboratory Methods
Levels of IGF-1 were measured by 2-site radioimmunometric assay (RIA) (Diagnostic Systems Laboratories Inc, Webster, Tex) (intra-assay coefficient of variation [CV], 4.93%). Levels of GH were measured by RIA (Corning Inc, Nichols Institute, San Juan Capistrano, Calif) (intra-assay CV, 3.33%; sensitivity, 0.01 ng/mL). Insulin levels were determined by RIA (Diagnostic Products Corp, Los Angeles, Calif) (intra-assay CV, 5.2%). Glucose, HbA1c, and lipid concentrations were measured by standard techniques.25 Concentrations of direct low-density lipoprotein cholesterol were determined by immunoseparation spectrophotometry (Specialty Laboratories, Santa Monica, Calif) (intra-assay CV, 3.0%). The CD4 cell count was performed using standard flow cytometry technology (B-D TruCount; Becton Dickinson, San Jose, Calif), and the HIV viral load was determined by ultrasensitive assay (Roche Amplicor HIV-1 Monitor Assay Version 1.0; Roche, Indianapolis, Ind), with a lower limit of detection of 50 copies/mL.
Pulsatility Analysis
Circulating GH levels in humans fluctuate widely due to pulsatile GH secretion by the pituitary gland. As a result, random measurement of serum GH will not establish accurate GH secretory patterns. We therefore used standard pulsatility techniques and sampling rates to determine the pattern of GH pulse secretion as has been previously established to assess GH pulsatility in response to GHRH administration.26-27 To assess growth hormone pulsatility we used Cluster28-29 and specified individual test-cluster sizes for the nadir and peak width of 2 (2 x 2).30
Lipodystrophy Rating Scales
Physicians and participants independently rated lipodystrophy in the face, abdomen, arms, and legs at the baseline and final visit using a 4-point rating scale (none, mild, moderate, or severe), and the changes in rating scores were compared between the groups. The patient rating scale was adapted from Lichtenstein et al.31-32 In contrast to prior studies using this scale, we limited our analysis to the face, arms, legs, and abdomen to allow assessment of the relative distribution of fat in the trunk and extremities in response to GHRH. Lipodystrophy ratings were performed by a single investigator (P.K.).
At the final visit, participants also completed a self-administered questionnaire regarding change in overall appearance over 3 months. Participants were asked if they believed their overall appearance to be much worse, somewhat worse, not different, somewhat better, or much better compared with 3 months previous. All questionnaires were completed at the final visit prior to unblinding of the study. Participants were not prompted or aided by the investigators in their answers.
Statistical Methods
The primary end point of the study was change in levels of IGF-1 between the groups. The study enrolled 31 men with a planned 10% dropout rate and was powered to detect a treatment difference of 105 ng/mL in IGF-1 level at a 2-sided P<.05 significance level, based on prior data showing the standard deviation of IGF-1 level in this population to be 87.0 ng/mL.12 The study was not powered in advance to detect changes in other metabolic variables but achieved statistical significance in many secondary end points (see "Results" section). Post hoc power analyses showed that the study was well powered to detect clinically relevant changes into the abnormal range in levels of glucose (91% power at P<.05) and HbA1c (99% power at P<.05) but may have been underpowered to detect significant changes in some variables. The t test was used to compare baseline data. Treatment effect was determined by comparison of change from baseline between treatment groups using the t test. For viral load and CD4 cell count, baseline values and the changes between treatment groups were compared with the Wilcoxon test. Race and overall antiretroviral use were compared using 2 likelihood ratios. The Fisher exact test was used for comparison of individual antiretroviral medications. All available data are included in the analyses in an intention-to-treat design. Similar results were obtained excluding the follow-up data from the 2 participants in the placebo group who completed the trial on an intention-to-treat basis (Grinspoon et al, unpublished data, February 2004). Also, we performed another analysis with the last observation carried forward for the 2 men who discontinued placebo injections and the results were again similar. Their treatment status was made available for safety reasons (because of adverse effects) (see "Results" section). In terms of missing data, we were unable to obtain follow-up data (and thus unable to calculate change from baseline) for 2 men, 1 in the treatment group and 1 in the placebo group. These men were unwilling or unable to make the follow-up visits (see "Results" section), and no interim data were available for them. We performed an analysis with the last observation carried forward for these 2 men and the results were nearly identical. P values remained significant for all the variables that are significant in the current analysis (Grinspoon et al, unpublished data, February 2004). Thus, these missing data do not skew the results, even with a conservative approach to impute missing data.
Outlier analysis was performed using the Dixon criterion.33 The randomization code was determined by the GCRC biostatistician using a permuted-block algorithm. All statistical analyses were performed using SAS JMP Statistical Database Software version 4 (SAS Institute Inc, Cary, NC). Statistical significance was defined as a 2-tailed value of P.05. Results are mean (SD) unless otherwise indicated.
Growth hormone-releasing hormone is generally well tolerated and adverse effects have been shown to occur in a minority of patients. Adverse effects known to occur rarely in response to GHRH administration include brief injection-site reactions, headache, flushing, dizziness, and urticaria.34 Approximately 60% of patients may develop anti-GHRH antibodies with no effect on response.35 With excess dosing, adverse effects similar to those observed with high-dose GH may occur, including arthralgias and fluid retention.36-37 In children, rare hypothyroidism can occur.35 An independent data and safety monitoring board, consisting of an AIDS expert, statistician, and community advocate, met every 3 months during the study to review adverse events.
|
|
| |
| |
|
|
|