| |
Combined Use of Rosiglitazone and Fenofibrate in Patients With Type 2 Diabetes
|
| |
| |
Prevention of Fluid Retention
Diabetes Jan 2007
Guenther Boden, Carol Homko, Maria Mozzoli, Meijuan Zhang, Karen Kresge, and Peter Cheung
From the Division of Endocrinology/Diabetes/Metabolism and the Clinical Research Unit, Temple University School of Medicine, Philadelphia, Pennsylvania
"....Compared with RGZ alone, RGZ/FFB was significantly more effective in lowering fasting plasma FFA levels and tended to be more effective in lowering A1C, fasting plasma glucose, and triglyceride levels, raising adiponectin levels and insulin sensitivity. Interestingly, RGZ/FFB completely prevented the water retention and weight gain associated with RGZ treatment. These preliminary results suggest that RGZ/FFB may be a more suitable treatment than RGZ to lower elevated plasma FFA levels long term and without rebound and to improve insulin resistance, glucose tolerance, and glycemic control in obese patients with type 2 diabetes...."
ABSTRACT
Elevated plasma free fatty acid (FFA) levels are responsible for much of the insulin resistance in obese patients with type 2 diabetes. To lower plasma FFA levels effectively and long term, we have treated eight obese patients with type 2 diabetes for 2 months with placebo followed by 2 months of treatment with a combination of rosiglitazone (RGZ) (8 mg/day) and fenofibrate (FFB) (160 mg/day) in a single-blind placebo-controlled study design. Compared with placebo, RGZ/FFB lowered mean 24-h plasma FFA levels 30% (P < 0.03) and mean 24-h glucose levels 23% (P < 0.03) and increased insulin-stimulated glucose uptake (glucose rate of disappearance [GRd], determined using euglycemic-hyperinsulinemic clamp) 442% (P < 0.01), oral glucose tolerance (area under the curve for 3-h oral glucose tolerance test) 28% (P < 0.05), and plasma adiponectin levels 218% (P < 0.01). These RGZ/FFB results were compared with results obtained in five patients treated with RGZ alone. RGZ/FFB prevented the fluid retention usually associated with RGZ (-1.6 vs. 5.6%, P < 0.05), lowered fasting plasma FFA more effectively than RGZ alone (-22 vs. 5%, P < 0.05), and tended to be more effective than RGZ alone in lowering A1C (-0.9 vs. -0.4%) and triglyceride levels (-38 vs. -5%) and increasing GRd (442 vs. 330%). We conclude that RGZ/FFB is a promising new therapy for type 2 diabetes that lowers plasma FFA more than RGZ alone and in contrast to RGZ does not cause water retention and weight gain.
Obesity causes insulin resistance; therefore, practically all obese people are insulin resistant in varying degrees (1,2). This is important because insulin resistance is not only a core pathogenetic abnormality of type 2 diabetes and the metabolic syndrome (3), but also plays a role in the development of atherosclerotic vascular disease (4). Whereas the mechanism by which obesity causes insulin resistance is still not entirely clear, there is strong evidence to suggest that elevated plasma free fatty acid (FFA) levels are responsible for much of the insulin resistance in obese people. The evidence can be summarized as follows: 1) plasma FFA levels are elevated in almost all obese people (3), 2) increasing plasma FFA levels increases insulin resistance dose dependently (5), and 3) decreasing plasma FFA levels decreases insulin resistance (6,7). Given that insulin resistance and elevated plasma FFA levels are at the core of serious health problems associated with obesity, it follows that elevated FFA levels should be a target of therapy. Indeed, lowering of plasma FFA levels into the normal range with acipimox, a nicotinic acid analog, has been shown to improve insulin resistance in obese patients with type 2 diabetes (6) and in first-degree relatives of patients with type 2 diabetes (7). It is, however, difficult to lower plasma FFA levels long term with currently available drugs. The use of nicotinic acid or of long-acting nicotinic acid analogs is associated with a rebound of plasma FFA to very high levels (8). This renders these drugs unsuitable for long-term control of plasma FFA. Thiazolidinediones (TZDs), a new class of blood glucose-lowering drugs, lower plasma FFA levels long term and without rebound (9-12). This effect, however, is modest (from <10% to about 20%) and sometimes absent (9-13). Thus, TZD-induced lowering of plasma FFA levels usually is not sufficient to maximally improve insulin sensitivity in patients with type 2 diabetes. Fibrates, another class of lipid-lowering drugs, also lower plasma FFA levels modestly and without rebound primarily by stimulating fat oxidation in the liver (13). As both classes of drugs work in different sites (TZDs primarily in fat and fibrates primarily in the liver) and through different mechanisms (TZDs through activation of peroxisome proliferator-activated receptor [PPAR]-y and fibrates through activation of PPAR-a) (13), their use in combination can be expected to have at least additive effects and hence should produce greater decreases in plasma FFA levels as well as greater improvements in insulin sensitivity than the use of either drug alone. This approach has been tried thus far only in one small study (14). In that study, treatment of healthy young men with rosiglitazone (RGZ) (8 mg/day) plus fenofibrate (FFB) (210 mg/day) for only 2 weeks lowered plasma FFA levels by about 40% (14). Therefore, the objective of the current study was to determine whether treating patients with type 2 diabetes with FFB plus RGZ (RGZ/FFB) lowered plasma FFA levels long term and improved insulin sensitivity more effectively than treatment with RGZ alone.
RESEARCH DESIGN AND METHODS
In the RGZ/FFB study, eight patients with type 2 diabetes were treated with RGZ plus FFB or placebo (Table 1). Of these, three were treated with metformin, three were treated with sulfonylureas and metformin, one received no blood glucose-lowering drugs, and one received insulin. These medications were withheld at least 72 h before and during hospital admissions but were otherwise continued throughout the studies. Informed consent was obtained from all subjects after explanation of the nature, purpose, and potential risks of the studies. The study protocol was approved by the institutional review board of Temple University Hospital.
In the RGZ study, five obese patients with type 2 diabetes were treated with RGZ (Table 1, lower panel). These patients were part of a previously published study (12) in which five patients were treated with RGZ (8 mg/day) and three with troglitazone. The study protocols were the same for both studies (2 months placebo followed by 2 months RGZ/FFB or RGZ).
Study volunteers were admitted to the clinical research center (CRC) at Temple University Hospital in the morning after an overnight fast. They underwent a thorough physical examination and had a 3-h oral glucose tolerance test (OGTT) with measurements of plasma glucose and insulin levels (RGZ/FFB study only). In addition, body composition was determined with bioelectric impedance analysis (15). The following day, an intravenous catheter was placed in one arm and used for blood sampling for 24-h blood profiles of glucose FFA and insulin (RGZ/FFB study only). Meals were served at 8:00 A.M., 12:00 P.M., and 6:00 P.M. On the next day, following completion of the 24-h blood sampling, a second intravenous catheter was placed in the other arm and the study volunteers underwent a 4-h one-stage euglycemic-hyperinsulinemic clamp. After that, they were discharged from the hospital and started either on RGZ (8 mg/day) and FFB (54 mg/day) or on placebo. RGZ, FFB, and placebo pills were ground up and administered in capsules to give the study a single-blind placebo-controlled design.
Outpatient visits.
The study volunteers were seen again as outpatients 1 week after discharge from the hospital. At that time, basal plasma FFA, glucose and insulin levels, and liver function tests were obtained and the FFB dose increased as needed (54 mg b.i.d. or t.i.d.) to bring plasma FFA levels into the 200-300 μmol/l target range. Three weeks after discharge from the hospital, this procedure was repeated, i.e., basal plasma FFA levels were determined and the FFB dose increased to 54 mg t.i.d. in all patients.
Second and third CRC admission.
After 2 months of treatment with placebo and again after 2 months of treatment with RGZ plus FFB (RGZ/FFB), all study volunteers were readmitted to the CRC and all tests that were done during the first admission were repeated. In between admissions two and three, all volunteers were seen as outpatients every 2-3 weeks.
Euglycemic-hyperinsulinemic clamping.
Regular human insulin was infused intravenously at a rate of 7 pmol • kg-1 • min-1 for 4 h. Plasma glucose concentrations were clamped at about 5.5 mmol/l by a feedback-controlled glucose infusion. Blood samples were obtained before (-180, -30, and 0 min) and at hourly intervals after insulin infusion for the determination of glucose and glycerol turnovers.
Indirect calorimetry.
Respiratory gas exchange was determined at 30-min intervals during the clamps with a metabolic measurement cart (DeltaTrac II; Sensormedics, Yorba Linda, CA) as previously described (16). Rates of protein oxidation were estimated from urinary nitrogen excretion with correction for changes in urine nitrogen pool size (17). Rates of protein oxidation were used to determine the nonprotein respiratory quotient. Rates of carbohydrate oxidation were determined with the tables of Lusk, which are based on a nonprotein respiratory quotient of 0.707 for 100% fat oxidation and 1.00 for 100% carbohydrate oxidation.
Glucose turnover.
Glucose turnover was determined with 6,62H2 glucose (Cambridge Isotope Labs, Andover, MA), which was infused intravenously for 7 h (from -3 to 4 h) starting with a bolus of 30 μmol followed by continuous infusion of 0.3 μmol • kg-1 • min-1. In hyperglycemic patients, the tracer infusion was adjusted to the degree of hyperglycemia. Glucose was isolated from blood for determination of 6,62H2 glucose enrichment (18). Rates of total body glucose appearance (GRa) and disappearance (GRd) were calculated using Steele's equation for non-steady state conditions (19). Endogenous glucose production (EGP) was determined by subtracting the glucose infusion rate (GIR) needed to maintain euglycemia from the rate of GRa.
Glycerol turnover.
2H5 glycerol (Cambridge Isotope Labs) dissolved in normal saline was infused from -90 until 240 min starting with a priming dose of 1.6 μmol/kg followed by a continuous infusion of 0.11 μmol • kg-1 • min-1. Blood for determination of 2H5 glycerol enrichment was collected at -180, -30, and 0 min and then at 30- to 60-min intervals until the end (240 min) of the clamp. Plasma was immediately separated at 4��C and stored at -70��C until analyzed. The trimethylsilyl derivative of glycerol was prepared as described previously (20). 2H5 glycerol enrichment was determined by gas chromatography-mass spectrometry (5989MS, 5890GC; Hewlett Packard, Palo Alto, CA) with the use of electron impact ionization and monitoring of ions at m/e 205 and 208. Glycerol Ra was calculated according to the equation of Steele corrected for the amount of exogenously infused stable isotope (18). Glycerol Ra = IE infusion/IE plasma -I/F, where Ra is the rate of appearance of glycerol (in μmol • kg-1 • min-1), IE infusion is the isotope enrichment of the infusate (atomic percent excess), IE plasma is the isotope enrichment of the plasma at isotopic equilibrium, and F is the isotope rate of infusion (in μmol • kg-1 • min-1). Glycerol Ra x 3 was assumed to reflect rates of whole-body lipolysis.
Analytical procedures.
Plasma glucose was measured with a glucose analyzer (YSI, Yellow Springs, OH). Insulin was determined in serum after protein precipitation with polyethylene glycol by radioimmunoassay with a specific antibody that cross-reacts minimally (<0.2%) with proinsulin (Linco, St. Charles, MO). Adiponectin was determined with a radioimmunoassay from Linco. Total plasma FFA was determined enzymatically in plasma containing EDTA and the lipoprotein lipase inhibitor Paroxam (0.25 mg/ml blood; Sigma, St. Louis, MO) with a kit from Wako (Richmond, VA).
Statistical analysis.
All data are expressed as means ± SE. Values between pre- and postplacebo and postdrug (either RGZ/FFB or RGZ) studies were compared using the paired Student's t test. Normality was tested with the Kolmogorov-Smirnov test. The Wilcoxon signed-rank test was used to determine significance if the data were not normally distributed. The pre- and postplacebo and posttreatment data were compared using a one-way repeated-measures ANOVA with the Student Newman-Keuls test used for multiple comparisons. The Friedman repeated-measures ANOVA on ranks was used when the data were not normally distributed. Data between the RGZ/FFB and RGZ studies were compared using the unpaired t test. Non-normal data were tested using the Mann-Whitney rank-sum test.
RESULTS
Body water and weight.
Placebo treatment had no effect on body water or on body weights in the RGZ/FFB and the RGZ study (Table 1). RGZ/FFB treatment also did not affect body water or body weight. RGZ treatment, in contrast, increased both body water (from 46.6 ± 3.6 to 49.4 ± 4.4 kg, P < 0.05) and body weight (from 100.9 ± 7.5 to 102.9 ± 7.9 kg, P < 0.05).
Twenty four-hour FFA and glucose profiles.
Twenty four-hour FFA and glucose profiles and mean 24-h FFA and glucose concentrations were lower post-RGZ/FFB than pre- and postplacebo (Fig. 1). Mean 24-h FFA levels decreased from 381 ± 44 and 384 ± 46 μmol/l (pre- and postplacebo, respectively) to 267 ± 22 μmol/l post-RGZ/FFB (-30%, P < 0.03). Mean 24-h plasma glucose concentrations decreased from 11.3 ± 1.8 and 12.3 ± 1.8 mmol/l (pre- and postplacebo) to 9.5 ± 1.7 mmol/l post-RGZ/FFB treatment (-23%, P < 0.03).
Clamp glucose and insulin levels.
Preclamp glucose concentrations were similar pre- and postplacebo (10.9 ± 1.9 vs. 10.1 ± 1.7 mmol/l, NS) but were lower post-RGZ/FFB treatment (8.0 ± 1.5 mmol/l, -21%, P < 0.05). During the last hour of the clamps, glucose concentrations were 5.9 ± 0.4, 5.8 ± 0.3, and 5.9 ± 0.2 mmol/l, respectively, pre- and postplacebo and post-RGZ/FFB (differences not statistically significant).
Preclamp insulin concentrations were 169 ± 44 and 133 ± 26 pmol/l pre- and postplacebo (NS) and decreased to 102 ± 19 pmol/l (-23%, P < 0.05) post-RGZ/FFB. During the last hour of the clamps, serum insulin concentrations were 706 ± 106, 648 ± 68, and 696 ± 86 pmol/l pre- and postplacebo and post-RGZ/FFB, respectively (differences not significant).
Glucose turnover
GIRs.
During the last hour of hyperinsulinemic clamping, GIRs needed to maintain euglycemia were 12.5 ± 3.0 and 14.0 ± 2.5 μmol • kg-1 • min-1 pre- and postplacebo, respectively (NS), but increased to 30.2 ± 5.5 μmol • kg-1 • min-1 (216%, P = 0.002) after RGZ/FFB (Fig. 2).
GRd.
GRd increased in response to hyperinsulinemia from 9.1 ± 0.9 to 17.5 ± 2.6 μmol • kg-1 • min-1 preplacebo (192%, P < 0.03), from 8.0 ± 0.4 to 14.0 ± 2.2 μmol • kg-1 • min-1 postplacebo (172%, P < 0.02), and from 6.9 ± 0.4 to 30.5 ± 5.3 μmol • kg-1 • min-1 post-RGZ/FFB (442%, P < 0.01). These results did not change significantly when the data were expressed as micromoles per kilograms of fat- free mass or micromoles per body surface area. The difference in insulin-stimulated GRd between postplacebo versus post-RGZ/FFB was highly significant (P = 0.004). Moreover, this difference entirely accounted for the postplacebo versus post-RGZ/FFB increase in GIR.
EGP.
EGP was 8.0 ± 0.8, 7.0 ± 0.4, and 6.0 ± 0.4 μmol • kg-1 • min-1 pre- and postplacebo and post-RGZ/FFB, respectively (differences not statistically significant). EGP was completely suppressed by insulin during the 1st hour of the clamps after RGZ/FFB but not after placebo (P < 0.02). After 2 h, EGP was equally suppressed in both groups (Fig. 2).
FFA, lipolysis, and FFA oxidation.
Preclamp basal FFA concentrations were significantly lower post-RGZ/FFB than postplacebo (423 ± 56 vs. 539 ± 39 μmol/l, -22%, P < 0.02) (Table 2). At the end of the 4-h clamps, plasma FFA concentrations decreased to 102 ± 25, 123 ± 26, and 86 ± 22 μmol/l pre- and postplacebo and post-RGZ/FFB, respectively (differences not statistically significant).
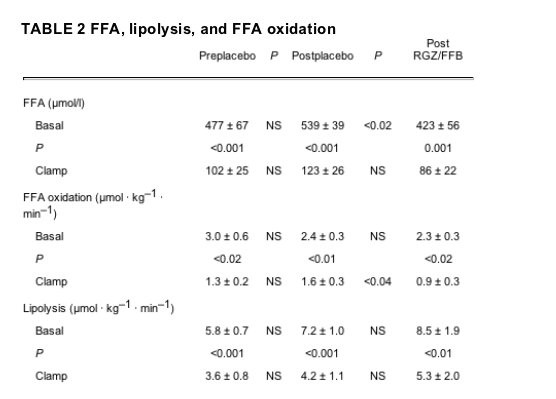
Neither placebo nor RGZ/FFB treatment had significant effects on basal rates of lipolysis or FFA oxidation. FFA oxidation, however, was more suppressed by insulin post-RGZ/FFB than postplacebo (1.6 ± 0.3 vs. 0.9 ± 0.3 μmol • kg-1 • min-1, P < 0.04).
OGTT.
Glucose tolerance was improved after RGZ/FFB (Fig. 3). The area under the curve for 3-h glucose tolerance was lower after RGZ/FFB than after placebo (32.8 vs. 45.3 mmol • 3 h-1 • l-1, P < 0.05), while serum insulin levels were similar.
Adiponectin.
Plasma adiponectin concentrations were suppressed pre- and postplacebo (4.1 ± 1.0 and 7.2 ± 1.5 ng/ml). Post-RGZ/FFB treatment, adiponectin concentrations rose from 7.2 ± 1.5 to 15.7 ± 2.8 ng/ml (P < 0.01).
Other effects.
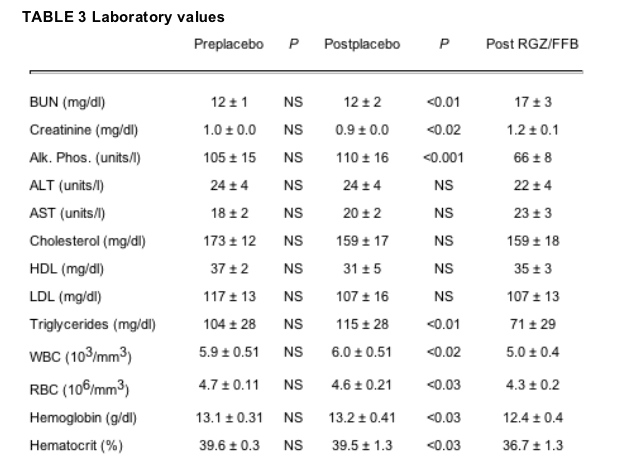
Both drugs were tolerated well, and none of the volunteers complained of fatigue or muscle or liver tenderness or pain (Table 3). The patients' weights did not change during the 2 months on placebo or RGZ/FFB (Table 1). Their blood urea nitrogen increased from 12 to 17 mg/dl and their serum creatinine from 1.0 to 1.2 mg/dl (Table 2). Liver function tests did not change except for the alkaline phosphatase, which decreased. There were also no significant changes in serum lipids except for triglyceride levels, which decreased from 115 to 71 mg/dl (-38%, P < 0.001) during RGZ/FFB treatment. RGZ/FFB was also associated with significant decreases in white and erythrocyte counts, hemoglobin, and hematocrit (Table 3).
Comparison between RGZ/FFB and RGZ alone.
Compared with placebo, body water did not change with RGZ/FFB (-1.6%, NS) but increased with RGZ (5.6%, P < 0.05) (Fig. 4 and Table 1). Basal plasma FFA levels decreased 22% (from 539 to 423 μmol/l, P < 0.02) with RGZ/FFB but did not change significantly with RGZ (617 ± 76 vs. 647 ± 76 μmol/l, NS). Thus, RGZ/FFB lowered plasma FFA significantly more than RGZ alone and prevented the RGZ-mediated water retention.
A1C decreased nonsignificantly from 8.0 ± 0.5 to 7.1 ± 0.4% (NS) in response to RGZ/FFB and from 8.7 ± 0.8 to 8.3 ± 0.8% (NS) in response to RGZ (Table 1). Basal plasma triglyceride levels decreased 38% (from 115 to 71 mg/dl, P < 0.001) after RGZ/FFB and 5% (from 187 ± 46 to 177 ± 61 mg/dl, NS) after RGZ. Basal plasma glucose levels decreased 21% (from 10.1 to 8.0 mmol/l, P < 0.05) after RGZ/FFB and 15% (from 9.2 ± 1.0 to 7.8 ± 1.0 mmol/l, NS) after RGZ. Plasma adiponectin levels more than doubled and became normal (from 7.2 ± 1.5 to 15.7 ± 2.8 μg/ml) after RGZ/FFB (P < 0.001), whereas after RGZ, adiponectin levels rose less and remained below normal (from 5.2 ± 0.5 to 9.6 ± 1.5 μg/ml, NS).
In the RGZ/FFB group, insulin-stimulated GRd rose 1.75-fold from 8.0 ± 0.4 to 14.0 ± 2.3 μmol • kg-1 • min-1 postplacebo (P < 0.01) and 4.2-fold from 6.9 ± 0.4 to 29.0 ± 5.2 μmol • kg-1 • min-1 post-RGZ/FFB (P < 0.003).
In the RGZ group, GRd rose 2.1-fold from 9.9 ± 1.5 to 18.7 ± 2.8 μmol • kg-1 • min-1 postplacebo (P < 0.05) and 3.3-fold from 9.5 ± 0.9 to 30.7 ± 8.3 μmol • kg-1 • min-1 post-RGZ/FFB (P < 0.005).
Comparing the effects of RGZ/FFB and RGZ, mean RGZ/FFB tended to be more effective in potentiating insulin-stimulated GRd (247 ± 55 vs. 115 ± 110%), but the difference was not statistically significant.
DISCUSSION
In this study, we tested a new method to lower plasma FFA levels in obese patients with type 2 diabetes consisting of treatment with a combination of a PPAR-y agonist (RGZ) and a PPAR-a agonist (FFB). The results showed that compared with placebo, RGZ/FFB treatment for 2 months reduced mean 24-h plasma FFA levels by about 30% (P < 0.03), normalized plasma adiponectin levels (from a depressed level of 7.2 to 15.7 ng/ml, P < 0.01), and more than doubled insulin-stimulated glucose uptake (from 14.0 to 30.4 μmol • kg-1 • min-1, P < 0.001).
The improved insulin sensitivity was accompanied by improved glucose tolerance. The area under the 3-h oral glucose tolerance curve decreased by 28% from 45.3 (after placebo) to 32.8 mmol/l x 3 h after RGZ/FFB (P < 0.05). Fasting plasma glucose levels decreased by 21% (from 10.1 to 8.0 mmol/l, P < 0.05) and mean 24-h glucose levels by 23% (from 12.4 to 9.5 mmol/l, P < 0.03).
To our knowledge, these are the first data obtained with combined use of a PPAR-y and a PPAR-a agonist (both marketed in the U.S.) in patients with type 2 diabetes. Dual PPARa-/y agonists have recently been developed by several pharmaceutical companies, and some are currently undergoing clinical trials (21,22). The results of these trials have not yet been published. These drugs all have fixed ratios of PPAR-y to PPAR-a activity. In contrast, by using RGZ and FFB, the y-to-a activity ratio can be varied by changing the doses of the two drugs. Whether this is an advantage over fixed ratio dual PPAR-y/a agents remains to be shown.
The mechanism by which RGZ/FFB lowered plasma FFA levels is not entirely clear. PPAR-y agonists increase FFA oxidation by direct action on fat and perhaps also in skeletal muscle as well as indirectly in skeletal muscle via induction of adiponectin expression in fat (rev. in 22). PPAR-a agonists, on the other hand, increase FFA oxidation primarily in the liver (13). Therefore, we expected RGZ/FFB to increase FFA oxidation rates. We were unable, however, to detect significant effects of RGZ/FFB on basal lipolysis and FFA oxidation rates (Table 2). On the other hand, insulin-induced suppression of FFA oxidation was greater after RGZ/FFB than after placebo (1.6 vs. 0.9 μmol • kg-1 • min-1, P < 0.04), indicating improved adipocyte insulin sensitivity. It seems possible, therefore, that the RGZ/FFB-mediated improved insulin sensitivity produced changes in FFA release (lipolysis) or FFA oxidation that were too small to be detected with our glycerol turnover method, but which, over a 2-month period of time, added up to a measurable decrease in plasma FFA levels. Alternatively, it is possible that RGZ/FFB increased FFA clearance by increasing FFA reesterification.
The mechanisms by which FFA can cause insulin resistance and by which a decrease in plasma FFA levels can improve insulin resistance have recently been investigated. These studies have shown that an increase in plasma FFA levels resulted in intramyocellular and intrahepatocellular accumulation of long-chain acyl-CoA and diacylglycerol and in activation of several serine/threonine kinases, including protein kinase and inhibitor of nuclear factor B kinase, followed by inhibition of insulin signaling, characterized by a decrease in tyrosine phosphorylation of insulin receptor substrate-1/2 and inhibition of phosphatidylinositol 3-kinase activation (23-25).
Adverse effects.
None of the study participants experienced muscle or hepatic tenderness, pain, fatigue, shortness of breath, or fluid accumulation. RGZ/FFB was associated with a significant increase in plasma creatinine from 0.9 to 1.2 mg/dl (P < 0.02). This, however, did not necessarily indicate a decrease in renal function, as FFB has been reported to increase creatinine production (26). RGZ/FFB also lowered white and erythrocyte counts. This effect is usually attributed to RGZ, which is known to promote hemodilution via PPAR-y-mediated renal sodium reabsorption (27). However, in our study, RGZ/FFB did not cause water retention; thus, the cause for the decrease in white and red cells remains uncertain. RGZ/FFB also did not affect plasma cholesterol or LDL or HDL levels. Therefore, whereas RGZ/FFB treatment appeared to be well tolerated, its long-term safety will need to be tested in a much larger number of patients and for a much longer period of time.
RGZ/FFB versus RGZ.
Both RGZ and FFB have been shown to increase FFA oxidation, decrease plasma FFA levels, and improve insulin sensitivity (9-14). By acting through different mechanisms (RGZ via PPAR-y and FFB via PPAR-a), and in different sites (RGZ mainly on fat and FFB mainly in the liver), we hypothesized that their actions would be at least additive and perhaps even synergistic. To test this hypothesis, we compared the RGZ/FFB results with RGZ data, which were part of a previously published study with eight patients with type 2 diabetes (12). Five of those patients received RGZ (8 mg/day) and three troglitazone. Only the five patients who received RGZ were used for comparison with RGZ/FFB. The results (Fig. 4) showed that RGZ/FFB lowered plasma FFA levels significantly more than RGZ. RGZ/FFB also tended to be more effective than RGZ in reducing insulin resistance, fasting plasma glucose, triglycerides, and A1C and in increasing plasma adiponectin levels. None of these differences, however, reached statistical significance, most likely because of the small sample number. Nevertheless, we believe that, collectively, these changes suggest that RGZ/FFB is superior to RGZ alone in improving insulin resistance and glycemic control in patients with type 2 diabetes, although this should be confirmed in a larger trial.
Body water and weight.
Many studies have shown that RGZ produces weight gain and water retention (rev. in 22). It was, therefore, surprising that neither body weight nor body water increased with RGZ/FFB (Table 1 and Fig. 4). These findings are supported, however, by several studies in rodents that have shown that FFB reduced or prevented weight gain induced by high-fat diets (28-30) or by RGZ (31,32). Our study is the first in humans or animals to show that RGZ/FFB prevented RGZ-induced fluid retention. The mechanism for this novel RGZ/FFB effect on water retention remains to be explored. It is, however, interesting that PPAR-a is highly expressed in kidneys, where it exerts many actions (33), and that PPAR-y present in renal collecting ducts is now believed to be responsible for TZD-mediated water retention (27). The observation that FFB prevented RGZ-induced water retention is of considerable clinical importance because it suggests that treatment with RGZ/FFB not only improves FFA levels, insulin resistance, and glycemic control more effectively than RGZ alone but that it may also prevent water retention, the most serious adverse effect associated with TZDs.
In summary, we have treated eight obese patients with type 2 diabetes for 2 months with placebo followed by 2 months of treatment with RGZ/FFB. RGZ/FFB was well tolerated and, compared with placebo, effectively lowered fasting and mean 24-h plasma FFA levels, decreased plasma triglyceride concentrations, increased plasma adiponectin levels, increased insulin sensitivity, and improved oral glucose tolerance and glycemic control. Compared with RGZ alone, RGZ/FFB was significantly more effective in lowering fasting plasma FFA levels and tended to be more effective in lowering A1C, fasting plasma glucose, and triglyceride levels, raising adiponectin levels and insulin sensitivity. Interestingly, RGZ/FFB completely prevented the water retention and weight gain associated with RGZ treatment. These preliminary results suggest that RGZ/FFB may be a more suitable treatment than RGZ to lower elevated plasma FFA levels long term and without rebound and to improve insulin resistance, glucose tolerance, and glycemic control in obese patients with type 2 diabetes.
This work was supported by National Institutes of Health Grants R01-DK-58895, R01-HL-733267, and R01-DK-066003 and a Mentor-Based Training Grant from the American Diabetes Association (all to G.B.).
|
|
| |
| |
|
|
|