| |
Comparison of Vildagliptin and Rosiglitazone Monotherapy in Patients With Type 2 Diabetes
|
| |
| |
Diabetes Care Feb 2007
A 24-week, double-blind, randomized trial
Julio Rosenstock, MD1, Michelle A. Baron, MD2, Sylvie Dejager, MD, PHD2, David Mills, MSC3 and Anja Schweizer, PHD3
1 Dallas Diabetes and Endocrine Center, Dallas, Texas
2 Novartis Pharmaceuticals, East Hanover, New Jersey
3 Novartis Pharma AG, Basel, Switzerland
ABSTRACT
OBJECTIVE-To compare the efficacy and tolerability of vildagliptin and rosiglitazone during a 24-week treatment in drug-naive patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS-This was a double-blind, randomized, active-controlled, parallel-group, multicenter study of 24-week treatment with vildagliptin (100 mg daily, given as equally divided doses; n = 519) or rosiglitazone (8 mg daily, given as a once-daily dose; n = 267).
RESULTS-Monotherapy with vildagliptin and rosiglitazone decreased A1C (baseline = 8.7%) to a similar extent during the 24-week treatment, with most of the A1C reduction achieved by weeks 12 and 16, respectively. At end point, vildagliptin was as effective as rosiglitazone, improving A1C by -1.1 ± 0.1% (P < 0.001) and -1.3 ± 0.1% (P < 0.001), respectively, meeting the statistical criterion for noninferiority (upper-limit 95% CI for between-treatment difference 0.4%). Fasting plasma glucose decreased more with rosiglitazone (-2.3 mmol/l) than with vildagliptin (-1.3 mmol/l). Body weight did not change in vildagliptin-treated patients (-0.3 ± 0.2 kg) but increased in rosiglitazone-treated patients (+1.6 ± 0.3 kg, P < 0.001 vs. vildagliptin). Relative to rosiglitazone, vildagliptin significantly decreased triglycerides, total cholesterol, and LDL, non-HDL, and total-to-HDL cholesterol (-9 to -16%, all P 0.01) but produced a smaller increase in HDL cholesterol (+4 vs. +9%, P = 0.003). The proportion of patients experiencing an adverse event was 61.4 vs. 64.0% in patients receiving vildagliptin and rosiglitazone, respectively. Only one mild hypoglycemic episode was experienced by one patient in each treatment group, while the incidence of edema was greater with rosiglitazone (4.1%) than vildagliptin (2.1%).
CONCLUSIONS-Vildagliptin is an effective and well-tolerated treatment option in patients with type 2 diabetes, demonstrating similar glycemic reductions as rosiglitazone but without weight gain.
Abbreviations: DPP-4, dipeptidyl peptidase IV · FPG, fasting plasma glucose · GLP, glucagon-like peptide · ITT, intention to treat · TZD, thiazolidinedione
INTRODUCTION
A promising new approach to treating type 2 diabetes is the augmentation of glucagon-like peptide (GLP)-1 receptor signaling by increasing endogenous GLP-1 through inhibition of the dipeptidyl peptidase IV (DPP-4) enzyme (1). Vildagliptin is a potent and selective DPP-4 inhibitor that improves islet function by increasing both - and β-cell responsiveness to glucose (2,3). Vildagliptin has been shown in 12-week studies to decrease A1C when given as monotherapy (4,5) or in combination with metformin (6).
Head-to-head comparison studies recently have been recommended to better establish the efficacy and safety of investigational therapies, such as vildagliptin monotherapy, relative to other current therapies (7). Several classes of drugs are approved for the pharmacological treatment of type 2 diabetes, including the thiazolidinediones (TZDs), rosiglitazone, and pioglitazone, which are among the most recent additions to the therapeutic armamentarium. Accordingly, the present multicenter, 24-week, double-blind, randomized, controlled clinical trial was conducted to compare the efficacy and tolerability of monotherapy with vildagliptin (100 mg daily) versus rosiglitazone (8 mg daily) in drug-naive patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS-
This was a 24-week, double-blind, randomized, active-controlled, parallel-group study conducted at 202 centers in 11 countries in the Americas and Europe. Eligible patients were randomized to receive vildagliptin 100 mg daily (given as equally divided doses) or rosiglitazone 8 mg daily (given as a once-daily dose) in a ratio of 2:1. Efficacy and tolerability were assessed at weeks 4, 12, 16, and 24 of active treatment.
The study enrolled type 2 diabetic patients with A1C in the range of 7.5-11.0%. These patients had received no pharmacologic treatment for at least 12 weeks before screening and no antidiabetic agent for >3 consecutive months at any time in the past and were considered to be representative of a drug-naive population. Male and female patients (nonfertile or of childbearing potential using a medically approved birth control method), aged 18-80 years, with BMI 22-45 kg/m2 and with fasting plasma glucose (FPG) <15 mmol/l were eligible to participate.
Patients were excluded if they had a history of type 1 diabetes or secondary forms of diabetes; acute metabolic diabetes complications; myocardial infarction, unstable angina or coronary artery bypass surgery within the previous 6 months; congestive heart failure; liver disease, such as cirrhosis or chronic active hepatitis; and any contraindications and warnings according to the country-specific label for rosiglitazone. The following laboratory abnormalities were also excluded: alanine aminotransferase or aspartate aminotransferase >2.5 times the upper limit of normal, direct bilirubin >1.3 times the upper limit of normal, serum creatinine levels >220 Êmol/l, clinically significant abnormal thyroid-stimulating hormone, or fasting triglycerides >7.9 mmol/l.
A1C, FPG, body weight, and vital signs were measured at each study visit. Standard hematology and biochemistry laboratory assessments were made at each visit except on week 16. Fasting lipid profiles were measured and electrocardiograms were performed at screening and at weeks 0, 12, and 24.
All adverse events were recorded. Edema was assessed by the investigator as part of the normal adverse event-reporting process, either as a new occurrence or worsening of an existing condition. Patients were provided with glucose-monitoring devices and supplies and instructed on their use. Hypoglycemia was defined as symptoms suggestive of low blood glucose confirmed by a self-monitored blood glucose measurement <3.1 mmol/l plasma glucose equivalent. Severe hypoglycemia was defined as any episode requiring the assistance of another party.
All laboratory assessments were made by central laboratories. All assessments, except A1C, were performed by BARC (Bioanalytical Research Corporation). Assays were performed according to standardized and validated procedures in accordance with good laboratory practice. A1C measurements were performed by either BARC-EU (Ghent, Belgium) for European patients or by Diabetes Diagnostics Laboratory (Columbia, MO) or Covance-US (Indianapolis, IN) for patients from the Americas. All samples from any single patient were measured by the same laboratory.
Analysis populations and data analysis
The primary intention-to-treat (ITT) population consists of all randomized patients who 1) had a screening A1C value 7.4%, 2) received at least one dose of study medication, and 3) had a baseline as well as at least one postbaseline A1C measurement. A total of 89 randomized patients were excluded from the primary ITT population for the following reasons: 4 received no intervention and 13 had no postbaseline A1C measurement; in addition, 61 patients were inappropriately randomized with screening A1C <7.4%, and 11 patients had no baseline A1C assessment due to a systematic error in the measurement of A1C by the U.S. laboratory originally used for the study. The U.S. laboratory was subsequently changed, and no measurements performed by the initial laboratory are used in the analyses. The statistical power of the study was preserved by recruitment of additional patients, and all samples from any single patient were measured by the same laboratory throughout the study. The safety population consists of all patients who received at least one dose of the study drug and had at least one postbaseline safety assessment.
The primary efficacy variable was the change from baseline in A1C at study end point using the last observation carried forward for patients who discontinued early. Secondary efficacy parameters included changes in FPG, fasting plasma lipids, and body weight. The efficacy analyses were performed with data from the primary ITT population, which was prespecified as the main efficacy population. Change from baseline in primary and secondary end points were analyzed using an ANCOVA model, with treatment and pooled center as the classification variables and baseline as the covariate. A test for the noninferiority of vildagliptin to rosiglitazone in A1C was carried out through a CI approach. Noninferiority for A1C was established if the upper limit of the 95% CI for the between-treatment difference in the adjusted mean change from baseline to end point obtained from the ANCOVA model did not exceed 0.4%. For the secondary efficacy variables, tests of superiority were conducted at the two-sided significance level of 0.05. In addition, prespecified subanalyses of A1C changes were conducted based on initial (baseline) A1C and on BMI category.
Ethics and good clinical practice
All participants provided written informed consent. The protocol was approved by the independent ethics committee/institutional review board at each study site, and the study was conducted in accordance with the Declaration of Helsinki, using Good Clinical Practice.
RESULTS-
A total of 786 patients were randomized, and 697 patients comprised the primary ITT population (459 patients randomized to receive vildagliptin 100 mg daily and 238 patients randomized to rosiglitazone 8 mg daily); 782 patients comprised the safety population. Figure 1 summarizes the disposition of patients from screening through study end point, and Table 1 reports the demographic and baseline metabolic characteristics of the patients in the primary ITT population. The groups were well balanced, with A1C averaging 8.7% and FPG averaging 10.3 mmol/l in both treatment groups. One-third of patients had an A1C >9%. Participants were predominantly Caucasian and obese (30% with BMI 35 kg/m2), with a mean age of 54 years and mean disease duration of 2.4 years. More than 85% of all patients randomized to either treatment completed the 24-week study.
Efficacy
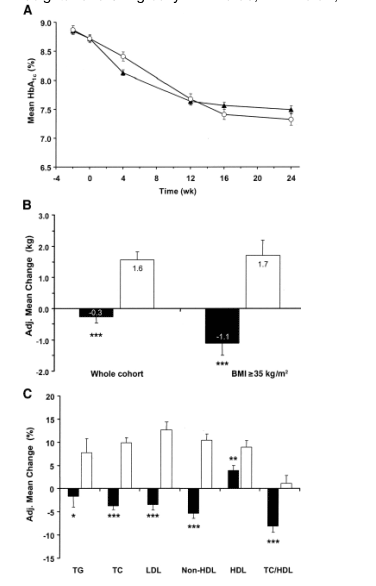
Figure 2A depicts the time-course of mean A1C during the 24-week treatment with vildagliptin 100 mg daily or rosiglitazone 8 mg daily. Baseline A1C values were identical in the two treatment groups (8.7 ± 0.1%). A1C decreased with vildagliptin treatment over the entire 24-week study period, with most of the reduction attained by week 12. Rosiglitazone treatment appeared to have a somewhat slower onset of effect, with nearly maximum reduction reached at week 16. In the primary ITT population, the adjusted mean change in A1C from baseline to study end point was -1.1 ± 0.1% (P < 0.001) in patients receiving vildagliptin (n = 459) and -1.3 ± 0.1% (P < 0.001) in patients receiving rosiglitazone (n = 238). Noninferiority of vildagliptin 100 mg daily to rosiglitazone 8 mg daily was established, as the upper limit of the 95% CI for the between-group difference in adjusted mean change in A1C (-0.01 to 0.39) did not exceed the prespecified noninferiority margin.
The decrease in A1C with either agent was substantially larger in patients with baseline A1C >9.0%, with mean A1C reductions of -1.8 ± 0.1% (P < 0.001) from a baseline of 10.0% (n = 166) with vildagliptin and of -1.9 ± 0.2% (P < 0.001) from a baseline of 9.9% (n = 88) with rosiglitazone. In the vildagliptin group, patients with BMI <30 kg/m2 showed a somewhat greater reduction in A1C (delta A1C = -1.3 ± 0.1%, n = 184) compared with obese patients with BMI 30 kg/m2 (delta A1C = -1.0 ± 0.1%, n = 275). Rosiglitazone, on the other hand, was somewhat more efficacious in patients with BMI >/=30 kg/m2 (delta A1C = -1.4 ± 0.1%, n = 155) than in leaner patients (delta A1C = -1.1 ± 0.2%, n = 83).
FPG also decreased significantly during the 24-week treatment with either agent. In the primary ITT population, the mean baseline FPG was 10.3 mmol/l in both treatment groups. The FPG reduction (adjusted mean change) was -1.3 ± 0.1 mmol/l (P < 0.001) in patients receiving vildagliptin and -2.3 ± 0.2 mmol/l (P < 0.001) in patients receiving rosiglitazone (P < 0.001 vs. vildagliptin).
Lipids and body weight
Figure 2 also depicts changes in body weight (B) and fasting lipid parameters (C) after the 24-week treatment with vildagliptin 100 mg daily or rosiglitazone 8 mg daily in the primary ITT population. Body weight at baseline averaged 91.2 ± 0.9 kg in the vildagliptin group and 93.1 ± 1.3 kg in the rosiglitazone group. Body weight did not change during 24-week treatment with vildagliptin but increased significantly during rosiglitazone monotherapy. The between-treatment difference in body weight was -1.9 ± 0.3 kg (P < 0.001). In addition, a post hoc analysis indicated that in the more severely obese population (BMI >/=35 kg/m2; mean body weight of about 111 kg; n = 208), a larger decrease in body weight was seen with vildagliptin, while the increase seen with rosiglitazone monotherapy was similar to the overall cohort. The between-treatment difference in body weight in this subpopulation was -2.8 ± 0.6 kg (P < 0.001).
In the primary ITT population, fasting lipid levels were similar in the two treatment groups at baseline, averaging 2.3 mmol/l for triglycerides, 5.3 mmol/l for total cholesterol, 3.1 mmol/l for LDL, 1.2 mmol/l for HDL, and 4.1 mmol/l for non-HDL cholesterol in the combined cohort, with a total-to-HDL cholesterol ratio of 4.7. Relative to rosiglitazone, vildagliptin produced significant decreases in triglycerides (-9%, P = 0.010) and total (-14%, P < 0.001), LDL (-16%, P < 0.001), and non-HDL cholesterol (-16%, P < 0.001) but less improvement in HDL cholesterol (+4 vs. +9% from baseline, P = 0.003 for between-group difference). Relative to rosiglitazone, vildagliptin decreased total-to-HDL cholesterol by 9.1 ± 1.9% (P < 0.0001).
Figure 2- A: Mean ± SE A1C during the 24-week treatment with vildagliptin (100 mg daily; black) or rosiglitazone (8 mg daily; white) in patients with type 2 diabetes (primary ITT population: vildagliptin, n = 434 at week -2, n = 397 at week 24; rosiglitazone, n = 221 at week -2, n = 209 at week 24). B: Adjusted mean change from baseline to end point in body weight in the primary ITT population and in subgroup of patients with BMI 35 kg/m2. black, vildagliptin 100 mg daily; white, rosiglitazone 8 mg daily. *P < 0.05; **P < 0.01; ***P < 0.001 vs. rosiglitazone. C: Adjusted mean change from baseline to end point in fasting lipid parameters in the primary ITT population. black, vildagliptin 100 mg daily; white, rosiglitazone 8 mg daily. *P < 0.05; **P < 0.01; ***P < 0.001 vs. rosiglitazone.
Tolerability
During the 24-week treatment, one or more adverse event was reported by 61.4% of patients receiving vildagliptin 100 mg daily and by 64.0% of patients receiving rosiglitazone 8 mg daily. In patients receiving vildagliptin, the most frequent specific adverse events (>/=4% in either group) were nasopharyngitis (6.8%), dizziness (6.0%), headache (5.0%), and upper respiratory tract infection (4.5%). In rosiglitazone-treated patients, the most common adverse events were nasopharyngitis (7.5%), headache (5.2%), dizziness (4.1%), and peripheral edema (4.1%). The incidence of peripheral edema with vildagliptin was 2.1%. Increased body weight was reported as an adverse event in 0.8% of vildagliptin-treated patients and in 2.6% of rosiglitazone-treated patients. One patient in each group reported one mild hypoglycemic event, and no serious hypoglycemic events occurred in either group.
The proportion of patients experiencing any serious adverse event in the two treatment groups was comparable (2.9 vs. 3.0%), and no specific serious adverse event was reported by more than one patient within a treatment group. The frequency of discontinuations due to adverse events was also similar in the vildagliptin (2.9%) and the rosiglitazone (3.4%) groups. There was one death during the study. This was a 70-year-old male subject randomized to vildagliptin who died from postsurgical complications.
With the exception of a slightly higher proportion of patients with notable hematocrit and hemoglobin abnormalities in the rosiglitazone group, there were no major changes from baseline to end point nor were there any between-treatment differences observed for any laboratory parameter or vital signs. The frequency of treatment-emergent electrocardiogram abnormalities was low and comparable in the two treatment groups.
CONCLUSIONS-
This study demonstrated that in patients representative of a drug-naive population, vildagliptin was well tolerated and caused no weight gain despite a significant and clinically meaningful decrease from baseline in A1C that was similar to that with rosiglitazone. As expected, both vildagliptin and rosiglitazone produced more substantial reductions in A1C in the subgroup of patients with a high baseline level, and as in the whole cohort, the improvement in glycemic control was similar in patients with high baseline A1C receiving vildagliptin (delta = -1.8%) or rosiglitazone (delta = -1.9%). Vildagliptin appeared to be slightly more effective than rosiglitazone in patients with BMI <30 kg/m2, and rosiglitazone was slightly more effective in obese patients (BMI >/=30 kg/m2).
Although the two agents had similar overall efficacy to reduce A1C, the different mechanism of action of the two agents likely underlies several differences noted regarding secondary efficacy end points as well as in tolerability profiles. Vildagliptin inhibits the enzyme DPP-4, causing an increase in active plasma levels of the incretin hormones GLP-1 and gastrointestinal polypeptide (3). Vildagliptin has been shown to improve islet function by increasing the ability of both a- and β-cells to sense and respond appropriately to glucose (2,8). These effects are thought to be mediated by GLP-1 (9). In contrast, the TZDs target insulin resistance acting by activation of peroxisome proliferator-activated y receptors, which results in enhanced peripheral and hepatic insulin action (10). Furthermore, TZDs stimulate differentiation of preadipocytes into new, small, and highly insulin-sensitive fat cells (10). This promotes storage of free fatty acids in adipose tissue, thus relieving the liver and muscle from lipotoxicity and reducing gluconeogenesis (11). The decrease in FPG in vildagliptin-treated patients seen in the present study was significantly less than that in patients receiving rosiglitazone, but the A1C improvements were similar, suggesting a more pronounced effect of vildagliptin on plasma glucose levels in the postprandial period and throughout the day.
Many effective antidiabetes agents lead to some weight gain as a result of improved glycemic control (12), and this is a particular limitation of (13) and potential safety concern about TZDs, due to their tendency to cause fluid retention and edema (14). In this study, the increase in body weight and incidence of edema in patients receiving rosiglitazone that was observed is consistent with previous reports, whereas vildagliptin achieved a comparable improvement in glycemic control with no weight gain and a low incidence of edema. The ability of vildagliptin to improve glycemic control without weight gain needs to be further clarified mechanistically.
In the present study, relative to rosiglitazone, vildagliptin treatment was associated with a significant improvement in triglycerides; total, LDL, and non-HDL cholesterol; and, importantly, the total-to-HDL cholesterol ratio. The changes in fasting lipids seen in rosiglitazone-treated patients were consistent with those reported in previous studies (15-17). The mechanism underlying the improvement in lipid profile seen in vildagliptin-treated patients is unknown but could reflect a chronic improvement in postprandial lipids, as suggested by a recent report that found decreased postprandial lipemia primarily through a reduction in intestinally derived apolipoprotein B-48-containing particles after a 4-week treatment with vildagliptin (8).
With the exception of a higher incidence of edema in rosiglitazone-treated patients, the two agents were similarly well tolerated in this 24-week study of vildagliptin 100 mg daily versus rosiglitazone 8 mg daily, and there was a very low incidence of hypoglycemia.
In conclusion, similar A1C efficacy can be achieved using the DPP-4 inhibitor vildagliptin or a TZD as monotherapy in drug-naive patients with type 2 diabetes. Vildagliptin is well tolerated, and, despite the improvement in glycemic control, it does not cause weight gain, which is an important consideration in the decision-making process for selecting first-line therapy in type 2 diabetes.
This study was funded by Novartis Pharmaceuticals. A list of investigators is provided in the APPENDIX.
The authors gratefully acknowledge the investigators and staff at the 202 participating sites and the editorial assistance of and helpful discussion with Beth Dunning Lower, PhD, who is a subcontractor for Novartis.
Footnotes
J.R. has served on advisory boards and as a consultant for Pfizer, Roche, Sanofi-Aventis, Novo Nordisk, MannKind, GlaxoSmithKline, Takeda, Centocor, Johnson & Johnson, Novartis, and Amylin; and has received grant/research support from Merck, Pfizer, Sanofi-Aventis, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, and Johnson & Johnson.
|
|
| |
| |
|
|
|