| |
Week 24 efficacy and safety of TMC114/ritonavir in treatment-experienced HIV patients POWER 2 Study
|
| |
| |
AIDS: Volume 21(6) 30 March 2007
Haubrich, Richarda; Berger, Danb; Chiliade, Philippec; Colson, Amyd; Conant, Marcuse; Gallant, Joelf; Wilkin, Timothyg; Nadler, Jeffreyh; Pierone, Geraldi; Saag, Michaelj; van Baelen, Benl; Lefebvre, Erick; on behalf of the POWER 2 Study Group
From the aUniversity of California San Diego, San Diego, California
bNorthstar Medical Center, Chicago, Illinois
cWhitman-Walker Clinic, Washington, DC
dBoston Community Research Initiative of New England, Boston, Massachusetts
eResearch Unit of Marcus Conant, San Francisco, California
fJohns Hopkins University School of Medicine, Baltimore, Maryland
gWeill Medical College of Cornell University, New York, New York
hUniversity of South Florida College of Medicine, Tampa, Florida
iTreasure Chest Infectious Disease Consultants, Vero Beach, Florida
jUniversity of Alabama, Birmingham, Alabama
kTibotec Inc., Yardley, Pennsylvania, USA
lTibotec BVBA, Mechelen, Belgium.
"....Despite previous exposure to a median of 11 ART and broad PI cross-resistance, 62% of patients who received TMC114/r 600/100 mg twice daily had ≥ 1.0 log10 copies/ml HIV-1 RNA reduction, and 39% had HIV-1 RNA < 50 copies/ml at week 24.... Results from this study and from POWER 1 suggest that TMC114/r provides a valuable treatment option for patients with significant ART resistance.....
....Subgroup analyses, albeit with small sample sizes, demonstrated that a greater number of active antiretroviral drugs (including NRTI and enfuvirtide) in the optimized background regimen was associated with better virological outcomes in all treatment groups. Enfuvirtide-naive patients receiving enfuvirtide had improved virological suppression rates....the incremental benefit of first-time enfuvirtide use in this study was more pronounced than was seen in the POWER 1 study, where patients had more favourable baseline characteristics...... For patients naive to Fuzeon 93% had a response using the 600/100 dose vs 45% who did not use Fuzeon.....Taken together, these findings underscore the value of using two or more active antiretroviral drugs in treatment-experienced patients and suggest a new treatment paradigm of achieving < 50 copies/ml in most patients with advanced HIV-1-treatment failure...."
Abstract
Background: Agents for the treatment of HIV-1-infected patients with resistance to current antiretroviral (ART) drugs are needed.
Methods: TMC114-C202 was a randomized, partially blinded, dose-finding study in treatment-experienced HIV-1-infected patients with one or more primary protease inhibitor (PI) mutations and HIV-1 RNA > 1000 copies/ml. Patients were randomized to receive one of four TMC114 doses given with ritonavir (TMC114/r) or investigator-selected control PI drug(s) (CPI); all received an optimized background regimen. The primary intent-to-treat analysis compared the proportion of patients achieving a ≥ 1 log10 copies/ml HIV-1 RNA reduction at week 24 between the treatment arms using the time-to-loss of virological response algorithm.
Results: For 278 patients at baseline, mean HIV-1 RNA was 4.7 log10 copies/ml, median CD4 cell count was 106 cells/μl; HIV-1 isolates had a median of three primary PI mutations and a median fold change in lopinavir susceptibility of 80. Discontinuation rates were 23% for TMC114/r versus 64% for CPI. More patients in each TMC114/r dose group achieved ≥ 1.0 log10 copies/ml reduction in HIV-1 RNA than in the CPI group (45-62% versus 14%; P ≦ 0.003): patients taking TMC114/r twice daily had the greatest responses. HIV-1 RNA was < 50 copies/ml in 18-39% of TMC114/r patients versus 7% CPI (P < 0.001 for highest dose). Mean CD4 cell count increased by 59-75 versus 12 cells/μl (TMC114/r versus CPI: P ≦ 0.005). Overall adverse event rates were similar in both arms, without significant differences among TMC114/r groups.
Conclusions: TMC114/r treatment resulted in greater virological and immunological responses in ART-experienced patients compared with CPI at 24 weeks.
Introduction
Antiretroviral drugs (ART) have revolutionized treatment for HIV-1 infection. Unfortunately, virological failure occurs in a significant proportion of patients and represents one of the most challenging HIV-1 management issues. Cohort studies suggest that approximately 10% of patients experience triple-class treatment failure, but the rate can be as high as 21% for those with prior dual agent treatment, and rates are increasing with time [1,2]. Patients with repeated episodes of virological failure frequently have drug resistance that limits treatment options [3]. Therefore, novel agents that are active against resistant virus are critically needed.
TMC114 (darunavir) is a new protease inhibitor (PI) with activity in vitro against both wild-type and multidrug-resistant HIV-1 strains [4]. POWER 2 (Performance of TMC114/r When Evaluated in Treatment-experienced Patients with PI Resistance; TMC114-C202) was designed to evaluate the efficacy and safety of TMC114 with ritonavir (TMC114/r) in four doses in patients who had experienced virological failure with three or more classes of ART drugs. Results from the primary 24-week analysis are reported.
Methods
Study design
POWER 2 was a multicentre, randomized, controlled, dose-finding phase IIb study conducted at 45 sites in the USA and Argentina. Before patient randomization, investigators selected an optimized background regimen and a protease inhibitor (PI) regimen based on the screening genotypic resistance data (VirtualPhenotype; Virco BVBA, Belgium) and prior treatment history. All patients received the optimized background regimen, which included at least two nucleoside analogue reverse transcriptase inhibitors (NRTI), with or without enfuvirtide, but which excluded non-nucleoside reverse transcriptase inhibitors (NNRTI). After stratification for HIV-1 RNA, inclusion of enfuvirtide and primary PI mutations (1, 2, and 3 or more) [5,6], patients were randomized centrally to the TMC114/r arm for one of four TMC114/r doses (400/100 mg or 800/100 mg once daily, 400/100 mg or 600/100 mg twice daily: blinded for dose, not schedule) or to investigator-selected control PI drug(s) (CPI arm). Patients randomized to the CPI arm changed their entire regimen at baseline; those randomized to TMC114/r switched their PI to TMC114/r for 2 weeks and their optimized background regimen thereafter.
Patients continued their regimen unless they were withdrawn because of virological failure or treatment-limiting toxicity. Confirmed virological failure was defined as HIV-1 RNA decrease of < 0.5 log10 copies/ml at week 8, < 1.0 log10 copies/ml at or beyond week 12 or an increase of 0.5 log10 copies/ml above nadir.
Patients underwent clinical and laboratory evaluations weekly for 4 weeks, then every 2 weeks until week 12, and every 4 weeks until week 24. HIV-1 RNA (Amplicor HIV-1 Monitor; Roche Molecular Systems, Branchburg, New Jersey, USA) and CD4 cell counts were performed at central laboratories. Phenotypic susceptibility was determined retrospectively (Antivirogram; Virco, BVBA, Mechelen, Belgium). Adverse events and laboratory abnormalities were categorized with a modified ACTG grading severity list.
After the primary analysis cut-off date (February 2005), all TMC114/r patients switched to TMC114/r 600/100 mg twice daily.
The study protocol was reviewed and approved by the appropriate institutional ethics committee(s) and health authorities, and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
Study population
Patients were HIV-1-infected adults with previous exposure to 3 months of two or more NRTI, one or more PI (on current PI for 12 weeks) and one or more NNRTI; their plasma HIV-1 RNA was > 1000 copies/ml and they had one or more primary PI mutation [5]. Prior enfuvirtide was allowed. Patients were excluded from the study if they had an active AIDS-defining illness, were taking investigational or disallowed medications, were pregnant or breast feeding, had evidence of active liver disease including hepatitis A, B or C, or had abnormal laboratory measurements.
Statistical methods
In the original protocol, the specified primary objective was to evaluate the dose-response relationship of different TMC114/r doses and the study had > 80% power to detect changes in HIV-1 RNA from baseline to week 24. After the planned interim analyses, the primary efficacy analysis was amended to compare the virological response between TMC114/r doses and the CPI arm using a confirmed HIV-1 RNA reduction from baseline of ≥ 1.0 log10 copies/ml at week 24 (time-to-loss of virological response). Secondary objectives included additional HIV-1 RNA, CD4 cell and safety parameters. Analyses were on an intent-to-treat basis and included all patients who took one or more dose of study medication. Patients who discontinued owing to toxicity, virological failure or loss to follow-up had their week 24 HIV-1RNA imputed as the baseline value (i.e. HIV-1 RNA change was zero). Following the planned interim analyses, efficacy data were censored in all patients who had not reached week 24 and were still receiving treatment; these patients did not contribute to the primary analysis.
Four pair-wise tests compared TMC114/r with CPI using a logistic regression model that included the stratification factors. The Bonferroni-Holmes method was used to adjust the overall nominal significance level for multiple comparisons. An O'Brien-Fleming alpha spending function was used to account for the fact that not all patients reached week 24. Thus, the alpha levels for the four comparisons to be considered significant were: < 0.004, 0.006, 0.008 and 0.0017. To test sensitivity of the imputation for time to loss of virological response, observed response rates as well as response rates using non-completer = failure and missing = failure imputations were derived.
Results
Study population
A total of 278 patients contributed to the primary analysis. No differences in baseline characteristics were noted among treatment groups (Table 1). As expected, patients were infected with highly resistant virus. Extensive phenotypic PI resistance was noted: median fold-change in effective drug concentration for PI other than TMC114 ranged from 24 to> 80, and 71% of patients had fold-change values above the cut-offs for seven other commercially available PI (tipranavir unavailable at this time). In contrast, the median TMC114 fold-change was 4.9. Patients in TMC114 & CPI arms had previously used 11 ART drugs 4 PIs and 5 NRTIs. Median HIV RNA was 4.6 log c/ml; median CD4 was 99 in TMC114 arm & 113 in CPI. 40% in TMC114 & 43% in CPI arm had CDC class C disease stage. 66-70% had 3 or more primary PI mutations. Primary duration of therapy: NRTIs 8 yrs, PIs 5 yrs. 4% had previously used tipranavir, and 23% Fuzeon.
The CPI arm included one (83%) or two (17%) PIs with ritonavir: (fos)amprenavir (42%), saquinavir (30%), lopinavir (28%) and atazanavir (11%). Within the CPI arm, 21% of the patients received a PI for which their HIV-1 had phenotypic sensitivity. The optimized background regimen contained one or more NRTI that retained activity against the patient's virus in 66% of patients. Enfuvirtide was included in the optimized background regimen of 120 (53%) TMC114/r recipients and 25 (47%) CPI recipients. Overall 28% (40/145) of patients receiving enfuvirtide had prior experience with it.
Patient disposition
Of 583 patients screened, 294 were randomized and 278 were included in the intent-to-treat analysis; 12% (7/60) of CPI recipients and 4% (9/234) of TMC114/r recipients were randomized but not treated, most (8) because consent was withdrawn. Overall, 31% of patients (85/278) discontinued: 23% (51/225) TMC114/r recipients and 64% (34/53) CPI recipients. Discontinuation in the CPI arm was mainly because of virological failure [25/53 patients (47%) versus 20/225 for TMC114/r (9%)].
When all patients were switched to TMC114/r 600/100 mg twice daily, 201 patients had reached week 24 or discontinued earlier; the remaining 77 were censored at their last visit. Hence, the primary efficacy analysis included 201 patients treated for ≥ 24 weeks.
HIV-1 RNA and CD4 cell responses at week 24
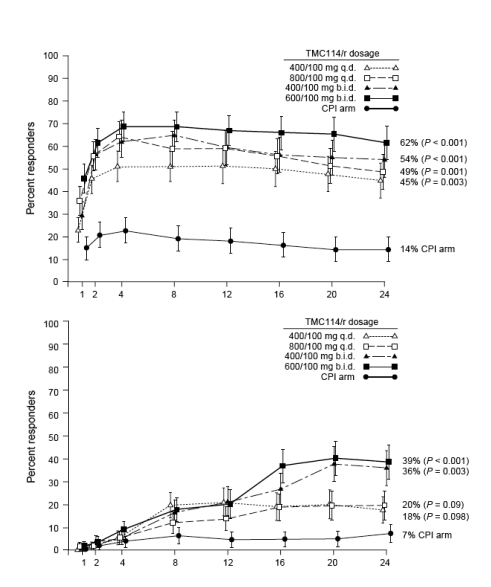
HIV-1 RNA reduction ≥ 1.0 log10 copies/ml was achieved by significantly more TMC114/r recipients [62% (24/39), 54% (21/39), 49% (20/41) and 45% (18/40) for patients receiving 600/100 mg twice daily, 400/100 mg twice daily, 800/100 mg once daily and 400/100 mg once daily, respectively] than CPI recipients [14% (6/42)] (P ≦ 0.003; Fig. 1a). Sensitivity analyses using observed data and non-completer = failure and missing = failure imputations showed similar differences between TMC114/r and the CPI arm (data not shown).
Mean reductions in log10 HIV-1 RNA were significantly greater at 1.7, 1.4, 1.3, and 1.2 copies/ml (highest to lowest TMC114/r doses) for the TMC114/r arm than for the CPI arm (0.3 copies/ml) (P ≦ 0.004).
The proportion of patients reaching HIV-1 RNA < 400 copies/ml was 49% of those taking either of the two TMC114/r twice daily doses, 30-34% of those taking either of the two TMC114/r once daily doses, and 10% of those taking CPI (P ≦ 0.013).
Significantly more TMC114/r recipients [twice daily dosages: 36-39% (P ≦ 0.003); once daily dosages: 18-20% (P ≦ 0.10)] achieved HIV-1 RNA < 50 copies/ml than did CPI recipients (7%; Fig. 1b). Sensitivity analyses demonstrated robust results that were independent of method. CD4 cell count increases (last observation carried forward) were higher for TMC114/r groups (59-75 cells/μl) than for the CPI arm (12 cells/μl) (P ≦ 0.005; Fig. 2).
Fig. 1. Week 24 virological response (intention-to-treat, time to loss of virological response). (a) Proportion of patients with HIV-1 RNA reduction ≥ 1.0 log10 copies/ml from baseline. (b) Proportion of patients with HIV-1 RNA < 50 copies/ml. P values are for comparisons with the control protease inhibitor (CPI) arm [investigator-selected control protease inhibitor drug(s)]. q.d., once daily; b.i.d., twice daily.
Effect of covariates on virological response
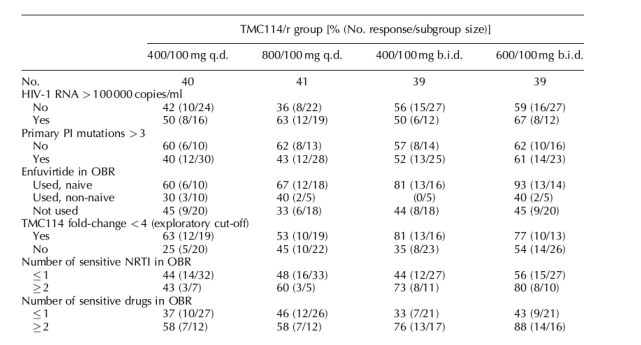
The rate of virological response (≥ 1 log10 copies/ml HIV-1 RNA decrease) did not appear to be affected by baseline HIV-1 RNA levels or number of primary PI mutations at baseline; response rates were similar among these respective subgroups in all TMC114/r dosage groups (Table 2). However, the small subgroup numbers limited statistical power. Greater susceptibility (using an exploratory cut-off of fold-change < 4 recommended by regulatory authorities) to TMC114 improved the virological response, although the effect was most noted at lower doses.
Inclusion of enfuvirtide and a greater number of sensitive NRTI or ART in the optimized background regimen markedly increased the proportion of patients having ≥ 1.0 log10 copies/ml reduction in HIV-1 RNA at week 24 (Table 2). A lower response rate was observed for patients with none or one sensitive ART in their optimized background regimen versus those with two or more. Patients using enfuvirtide for the first time had a greater response than those not receiving enfuvirtide.
Table 2. Percentage of patients with HIV-1 RNA reduction ≥ 1 log10 copies/ml at week 24 by stratification and baseline subgroups.
Regardless of baseline viral load being above or below 100,000 responses (>1 log VL reduction) were equally good. Actually some with >100,000 appeared to do better. Of note in the CPI group patients with >100,000 VL at baseline had half the response rate. Using the 600/100 dose the same percent had a viral response regardless of whether baseline VL was below or above 100,000-61%. The response rate was much better for patients using Fuzeon. For patients naive to Fuzeon 93% had a response using the 600/100 dose vs 45% who did not use Fuzeon. For patients using 2 or more sensitive NRTIs 80% had response vs 56% who used 1 or less sensitive NRTI. For patients with 2 or more sensitive drugs in regimen 88% had response vs 43% who had 1 or less sensitive drugs.
Safety profile
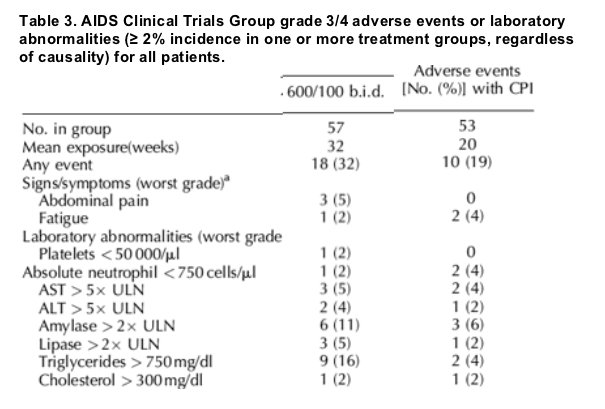
Through 24 weeks, the incidence of adverse events of all grades regardless of causality was comparable between TMC114/r and CPI arms. No relationship with TMC114/r dose was observed for any adverse event. When excluding enfuvirtide-associated injection-site reactions, the following adverse events (of any grade, any causality and occurring in ≥ 10% of patients) occurred mostly at similar rates in the TMC114/r treatment groups and CPI arm: headache, nausea, diarrhea, fatigue, upper respiratory tract infection, insomnia and pyrexia. Mean exposure to study medication was longer for the TMC114/r arm than for the CPI arm (32 versus 20 weeks). There was a limited number of individual grade 3 or 4 adverse events recorded (Table 3).
Adverse events led to treatment discontinuation in 8% (18/225) of TMC114/r recipients and 4% (2/53) of CPI recipients. The discontinuation rate was similar in all TMC114/r groups (7-9%), and all events were isolated except two cases each of elevated lipase and gamma-glutamyl transpeptidase. Overall, 15% (33/225) of TMC114/r and 8% (4/53) of CPI patients reported one or more serious adverse event, the most common being pneumonia (in 2% and 4% of TMC114/r and CPI arms, respectively). Occurrences of serious adverse events ranged from 9% (5/57) with 600/100 mg twice daily group to 23% (13/56) with 800/100 mg once daily and were not dose related. Six TMC114/r patients died during the study. All deaths were considered by the investigator to be unrelated (five) or doubtfully related (one) to study medication.
Grade 3 or 4 triglyceride elevations were more common in the two groups receiving TMC114 twice daily than in the other groups (Table 3). However, mean triglyceride levels decreased in all treatment groups. Week-24 triglycerides reductions were 0.6-2.0 mmol/l for the TMC114/r groups compared with 0.5 mmol/l for the CPI group. Collection of long-term safety data from this study is ongoing.
Discussion
Despite previous exposure to a median of 11 ART and broad PI cross-resistance, 62% of patients who received TMC114/r 600/100 mg twice daily had ≥ 1.0 log10 copies/ml HIV-1 RNA reduction, and 39% had HIV-1 RNA < 50 copies/ml at week 24. In contrast, 14% and 7%, respectively, of CPI recipients achieved the same endpoints. These differences were statistically significant and are clinically relevant. The study confirmed a dose-response relationship for virological efficacy with TMC114/r. These results, plus those from pharmacokinetic/pharmacodynamic analyses (pooled POWER 1 and 2) [7], validate the selection and regulatory approval of TMC114/r 600/100 mg twice daily for use in treatment-experienced patients.
Advances in ART therapy have led to the availability of safe, convenient and effective treatments capable of reducing HIV-1 RNA to < 50 copies/ml in most treatment-naive individuals [8]. Virological suppression after virological failure of the initial regimen remains achievable [9], but options for more drug-experienced patients are limited owing to cross-resistance. For patients with extensive drug resistance, use of enfuvirtide, combined with existing NRTI and PI, produced improved responses in another trial [10], but the maximal viral suppression rate remained suboptimal. Results from this study and from POWER 1 [11] suggest that TMC114/r provides a valuable treatment option for patients with significant ART resistance.
Subgroup analyses, albeit with small sample sizes, demonstrated that a greater number of active antiretroviral drugs (including NRTI and enfuvirtide) in the optimized background regimen was associated with better virological outcomes in all treatment groups. Enfuvirtide-naive patients receiving enfuvirtide had improved virological suppression rates. Overall responses to CPI regimens containing enfuvirtide were low. The incremental benefit of first-time enfuvirtide use in this study was more pronounced than was seen in the POWER 1 study, where patients had more favourable baseline characteristics [11]. Taken together, these findings underscore the value of using two or more active antiretroviral drugs in treatment-experienced patients and suggest a new treatment paradigm of achieving < 50 copies/ml in most patients with advanced HIV-1-treatment failure.
In this 24-week analysis, incidence of adverse events was similar with the TMC114/r and CPI arms, even though the drug exposure was shorter with the CPI arm. Despite this potential bias against TMC114/r, adverse events were similar for all treatments. No differences in the incidence of laboratory abnormalities were noted, nor was there a dose-response relationship for adverse events or laboratory abnormalities. Therefore, the improved HIV-1 RNA reduction with higher doses of TMC114/r did not occur at the expense of patient safety and therapy tolerability.
In conclusion, the combination of TMC114/r 600/100 mg twice daily with an optimized background regimen over 24 weeks was effective for these treatment-experienced HIV-1-infected patients whose virus carried multiple PI-resistance mutations. These data suggest that TMC114/r will help to bridge the gap between the different treatment goals defined for treatment-experienced and treatment-naive patients, moving from previously accepted goals of partial and transient HIV-1 RNA reduction, with maintenance of immunological function, to a more effective and durable strategy of achieving complete viral suppression.
|
|
| |
| |
|
|
|