| |
Purified Fish Oil+Statin Reduces Risk for Heart Disease in Japan More Than Statin Alone
|
| |
| |
"Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis"
The Lancet 2007; March 31, 369:1090-1098
Dr Mitsuhiro Yokoyama MD a , Hideki Origasa PhD b, Masunori Matsuzaki MD c, Yuji Matsuzawa MD d, Yasushi Saito MD e, Yuichi Ishikawa MD f, Shinichi Oikawa MD g, Jun Sasaki MD h, Hitoshi Hishida MD i, Hiroshige Itakura MD j, Toru Kita MD k, Akira Kitabatake MD l, Noriaki Nakaya MD m, Toshiie Sakata MD n, Kazuyuki Shimada MD o and Kunio Shirato MD p, for the Japan EPA lipid intervention study (JELIS) Investigators
Purified fish oil+statin.....resulted in a 19% relative reduction in major coronary events (p=0·011) compared to a statin alone.....Unstable angina and non-fatal coronary events were also significantly reduced in the EPA group (fish oil+statin). Sudden cardiac death and coronary death did not differ between groups. In patients with a history of coronary artery disease who were given EPA treatment, major coronary events were reduced by 19% (secondary prevention subgroup: 158 [8·7%] in the EPA group vs 197 [10·7%] in the control group; p=0·048)....
.....EPA ethyl ester, which is purified from n-3 polyunsaturated fatty acids present in fish oil, is approved by Japan's Ministry of Health, Labour, and Welfare as a treatment for hyperlipidaemia and peripheral artery disease..... The Japan EPA Lipid Intervention Study (JELIS) tests the hypothesis that long-term use of EPA is effective in reduction of major coronary events in Japanese... Patients were randomly assigned to receive either 1800 mg of EPA daily with statin (EPA group; n=9326) or statin only (controls; n=9319) with a 5-year follow-up.....hypercholesterolaemic patients given statins..... Total and LDL cholesterol at the last clinic visit decreased significantly by 19% and 25% from baseline in both groups, respectively. Triglyceride decreased significantly by 9% from baseline in the EPA group and by 4% in controls (p<0·0001 between groups)..... Rates of discontinuation because of treatment-related adverse events were 1087 (11·7%) in the EPA group and 673 (7·2%) in the control group (p<0.0001). Most adverse effects attributable to EPA allocation were regarded as mild. The following factors were more common in the EPA group than in controls: abnormal laboratory data; gastrointestinal disturbances such as nausea, diarrhoea, or epigastric discomfort; skin abnormalities such as eruption, itching, exanthema, or eczema; and haemorrhages such as cerebral and fundal bleedings, epistaxis, and subcutaneous bleeding. The frequency of new cancers did not differ....
"....Our study has some specific characteristics. First, we used highly purified EPA rather than n-3 polyunsaturated fatty acids or fish oils. This trial is a pharmacological intervention rather than a food-based or nutrient trial..... Our findings accord with a cohort study by the Japan Public Health Centre, which used a food-frequency questionnaire.31 Iso and co-workers31 reported that, compared with a small intake of fish (once a week or about 20 g per day), a high intake (eight times per week, or about 180 g per day) was associated with a substantially reduced risk of coronary heart disease, especially non-fatal cardiac events, in middle-aged Japanese men and women....our population was exclusively Japanese. In Japan, death from coronary artery disease is rare and the average dietary intake of fish is about five times higher than that in other countries... The beneficial effects of EPA could have stemmed from many biological effects that lead to the attenuation of thrombosis, inflammation, and arrhythmia in addition to a reduction of triglycerides. Overall, this study shows that EPA, at a dose of 1800 mg per day, is a very promising regimen for prevention of major coronary events, especially since EPA seems to act through several biological mechanisms. Because our population was exclusively Japanese, we cannot generalise our results to other populations. We need to investigate whether EPA is effective for prevention of major coronary events in hypercholesterolaemic patients without or with coronary artery disease in other countries...."
Summary
Background
Epidemiological and clinical evidence suggests that an increased intake of long-chain n-3 fatty acids protects against mortality from coronary artery disease. We aimed to test the hypothesis that long-term use of eicosapentaenoic acid (EPA) is effective for prevention of major coronary events in hypercholesterolaemic patients in Japan who consume a large amount of fish....patients in this study received either EPA or a statin+EPA in control group.... The 5-year cumulative rate of major coronary events was 2·8% in the EPA group and 3·5% in controls, resulting in a significant relative risk reduction of 19% in the EPA group (p=0·011)
Methods
18 645 patients with a total cholesterol of 6·5 mmol/L or greater were recruited from local physicians throughout Japan between 1996 and 1999. Patients were randomly assigned to receive either 1800 mg of EPA daily with statin (EPA group; n=9326) or statin only (controls; n=9319) with a 5-year follow-up. The primary endpoint was any major coronary event, including sudden cardiac death, fatal and non-fatal myocardial infarction, and other non-fatal events including unstable angina pectoris, angioplasty, stenting, or coronary artery bypass grafting. Analysis was by intention-to-treat.
Findings
At mean follow-up of 4·6 years, we detected the primary endpoint in 262 (2·8%) patients in the EPA group and 324 (3·5%) in controls-a 19% relative reduction in major coronary events (p=0·011). Post-treatment LDL cholesterol concentrations decreased 25%, from 4·7 mmol/L in both groups. Serum LDL cholesterol was not a significant factor in a reduction of risk for major coronary events. Unstable angina and non-fatal coronary events were also significantly reduced in the EPA group. Sudden cardiac death and coronary death did not differ between groups. In patients with a history of coronary artery disease who were given EPA treatment, major coronary events were reduced by 19% (secondary prevention subgroup: 158 [8·7%] in the EPA group vs 197 [10·7%] in the control group; p=0·048). In patients with no history of coronary artery disease, EPA treatment reduced major coronary events by 18%, but this finding was not significant (104 [1·4%] in the EPA group vs 127 [1·7%] in the control group; p=0·132).
Interpretation
EPA is a promising treatment for prevention of major coronary events, and especially non-fatal coronary events, in Japanese hypercholesterolaemic patients.
Introduction
Epidemiological and clinical evidence suggests a significant inverse association between long-term intake of long-chain n-3 polyunsaturated fatty acids, especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and mortality associated with coronary artery disease.1-7 Thus, the consumption of fish or fish-oil could protect against major events associated with coronary artery disease, especially fatal myocardial infarction and sudden cardiac death. Two large-scale secondary prevention trials, the Diet and Reinfarction Trial and the Gruppo Italiano per lo Studio della Sopravivenza nell' Infarto Miocardico-Prevenzione Trial, reported that increased consumption of fish or fish-oil supplements reduced coronary death in postinfarction patients.8,9 No randomised trials have examined the effects of n-3 polyunsaturated fatty acids on major coronary events in a high-risk, primary prevention population.
EPA ethyl ester, which is purified from n-3 polyunsaturated fatty acids present in fish oil, is approved by Japan's Ministry of Health, Labour, and Welfare as a treatment for hyperlipidaemia and peripheral artery disease. The biological functions of EPA include reduction of platelet aggregation,10,11 vasodilation,12,13 antiproliferation,14 plaque-stabilisation,15 and reduction in lipid action.16,17 Therefore the preventive effects of EPA on major cardiovascular events are of both clinical interest and therapeutic importance.
Primary and secondary prevention trials have proved that cholesterol-lowering treatment with inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase-statins-reduces the risk of all-cause mortality and major cardiovascular events in patients with a wide range of cholesterol concentrations, whether or not they have had coronary artery disease.18-21 Thus, statins are now established as the first-line treatment for hyperlipidaemia.22 Preliminary data for treatment with a combination of n-3 polyunsaturated fatty acids and statins have shown beneficial effects on the lipid profiles of patients with a mixed type of hyperlipidaemia;23-25 however, no major long-term interventional trial has yet investigated whether the addition of EPA to conventional statin treatment would yield an incremental clinical benefit. The Japan EPA Lipid Intervention Study (JELIS) tests the hypothesis that long-term use of EPA is effective in reduction of major coronary events in Japanese hypercholesterolaemic patients given statins.
Results
Patients were monitored for an average of 4·6 years (SD 1·1). Table 1 shows baseline characteristics of the treatment groups. The mean age of all patients was 61 years and 12 786 patients (69%) were women. Mean concentrations of total cholesterol and triglyceride were 7·1 mmol/L and 1·7 mmol/L; and mean LDL and HDL cholesterol concentrations were 4·7 mmol/L and 1·5 mmol/L, respectively. The webtable shows baseline characteristics for primary and secondary prevention subgroups. Of 3664 patients with documented coronary artery disease, 1050 had a history of myocardial infarction, 2903 of angina pectoris, and 895 angioplasty, stenting, or coronary artery bypass grafting.
Average doses were pravastatin 10·0 mg daily (SD 9·1) and simvastatin 5·6 mg daily (1·8). 16 449 (90%) patients took 10 mg pravastatin or 5 mg simvastatin. The 5-year follow-up rate was 16 971 (91%). Similar proportions of participants remained compliant in each treatment group. Study drug regimens were maintained until trial termination by 6151 (73%) of controls and in the treatment group 5883 (71%) of patients continued to take EPA and 6136 (74%) continued to take statin.
586 patients (262 assigned to EPA and 324 controls) reached the composite primary endpoint. Figure 2 shows Kaplan-Meier curves for the primary endpoint. The 5-year cumulative rate of major coronary events was 2·8% in the EPA group and 3·5% in controls, resulting in a significant relative risk reduction of 19% in the EPA group (p=0·011). Figure 3 shows that EPA treatment was associated with a significant reduction of 24% in the frequency of unstable angina. The occurrence of coronary death or myocardial infarction was not significantly lower (22%) in the EPA group than in controls. The frequency of fatal or non-fatal myocardial infarction was not significantly reduced (23%) in the EPA group; however, that of non-fatal coronary events (including non-fatal myocardial infarction, unstable angina, and events of angioplasty, stenting, or coronary artery bypass grafting) was significantly lower (19%) in the EPA group than in controls.
Table 2 sets out major coronary events in the two treatment groups for comparison with specific background characteristics of all populations. For example, we grouped patients according to their LDL cholesterol at baseline. The relative reduction in major coronary events risk in the EPA group was of a similar magnitude in patients with different ranges of LDL cholesterol values, suggesting that LDL cholesterol is not an important factor in reduction of risk for major coronary events.
In the primary prevention subgroup, EPA treatment was associated with a non-significant 18% reduction in major coronary events. Figure 3 shows the non-significant reductions of 18%, 21%, and 20% in coronary death or non-fatal myocardial infarction, fatal or non-fatal myocardial infarction, and non-fatal coronary events, respectively. In the secondary prevention subgroup, allocation to the EPA treatment was associated with a significant 19% reduction in major coronary events. EPA treatment was also associated with a significant 28% reduction in the incidence of unstable angina. This treatment also produced non-significant reductions of 25%, 25%, and 18% in coronary death or myocardial infarction, fatal or non-fatal myocardial infarction, and non-fatal coronary events, respectively.
In the other analyses, stroke occurred in 162 (1·7%) controls and 166 (1·8%) patients given EPA. Figure 3 shows that the frequency of ischaemic and haemorrhagic strokes did not differ between the two treatment groups, and neither did all-cause mortality.
Figure 4. Percentage changes from baseline in serum lipid profile
TC=total cholesterol. LDL C=low-density lipoprotein cholesterol. HDL C=high-density lipoprotein cholesterol.
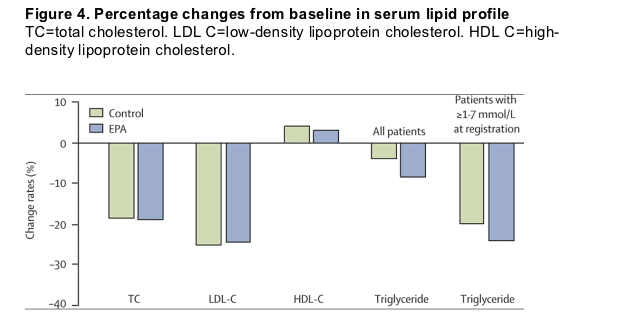
Figure 4 summarises the change in lipid values after treatment. Total and LDL cholesterol at the last clinic visit decreased significantly by 19% and 25% from baseline in both groups, respectively. Triglyceride decreased significantly by 9% from baseline in the EPA group and by 4% in controls (p<0·0001 between groups). Both treatments produced only small changes in HDL cholesterol. The fatty acid concentrations at baseline were the average values for all patients who gave informed consent in the control group (n=8076) and the EPA group (n=8321). Plasma EPA at baseline was 2·9% of total molecules of fatty acids (mol %). To assess the effect of EPA treatment, plasma fatty acid values were compared for all patients who were still compliant after 5 years of observation (controls: n=4854, EPA group: n=4970). Plasma EPA concentration and the ratio of EPA to arachidonic acid at baseline were 93 mg/L and 0·60 in controls, and 97 mg/L and 0·63 in the EPA group, respectively. Plasma EPA concentration and the ratio of EPA to arachidonic acid at year 5 were 93 mg/L and 0·59 in controls. On the other hand, plasma EPA concentration at year 5 was 169 mg/L in the EPA group, which was a 70% increase from baseline. The ratio of EPA to arachidonic acid increased two-fold from 0·63 to 1·23 in the EPA group. Similar results were reported previously.11,28
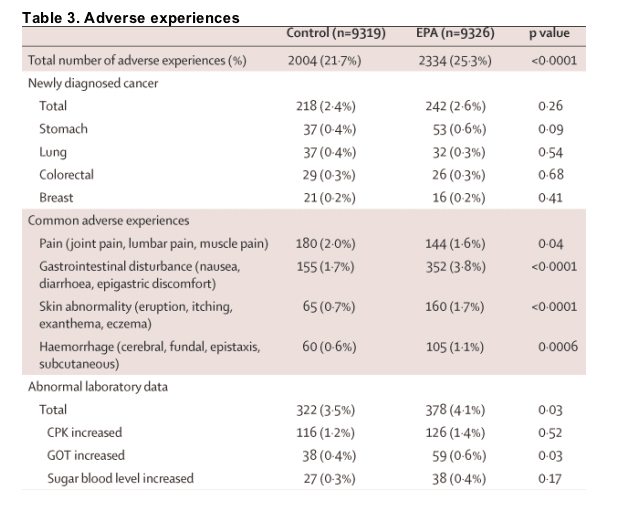
Table 3 shows that a quarter of patients in the EPA group had adverse experiences related to treatment, compared with about a fifth of controls. Rates of discontinuation because of treatment-related adverse events were 1087 (11·7%) in the EPA group and 673 (7·2%) in the control group. Most adverse effects attributable to EPA allocation were regarded as mild. The following factors were more common in the EPA group than in controls: abnormal laboratory data; gastrointestinal disturbances such as nausea, diarrhoea, or epigastric discomfort; skin abnormalities such as eruption, itching, exanthema, or eczema; and haemorrhages such as cerebral and fundal bleedings, epistaxis, and subcutaneous bleeding. The frequency of new cancers did not differ.
|
|
| |
| |
|
|
|