| |
HIV Infection Appears To Increase Risk Of Heart Attack: doubled risk in HIV+ vs HIV-negative in Mass Gen Hosp study
|
| |
| |
HIV and Heart Attack
http://www.emaxhealth.com
Researchers from Massachusetts General Hospital have found that infection with HIV, the virus that causes AIDS, is also associated with increased risk of myocardial infarction or heart attack.
While rates of several cardiovascular risk factors were also increased in study participants infected with HIV, the increased incidence of heart attack was beyond what could be explained by risk factor differences. The report will be published in the Journal of Clinical Endocrinology and Metabolism and has been released online.
"Our study shows a higher incidence of myocardial infarction and major cardiovascular risk factors in HIV-infected patients, compared with noninfected patients," says Steven Grinspoon, MD, of the MGH Program in Nutritional Metabolism and Neuroendocrine Unit, the report's senior author. "Those findings indicate that those infected with HIV should be assessed for cardiovascular risk factors and that we urgently need to develop strategies to modify those risks."
It has been recognized that many HIV-infected individuals have metabolic abnormalities - including altered levels of blood lipids such as cholesterol, insulin resistance, type 2 diabetes, and changes in fat distribution in the body. Researchers have reported that patients taking antiretroviral medications may have increased risk of heart attacks, but few studies have directly examined whether HIV-infected patients in general have more heart attacks than non-infected individuals do.
The researchers took advantage of the Research Patient Data Registry, a database of demographic and diagnostic information on more than 1.7 million patients treated at MGH and Brigham and Women's Hospital since 1993. They compared information on almost 4,000 HIV-infected patients with data from more than one million patients without HIV. Study participants were aged 18 to 84 and were seen at least twice during the study period of almost eight years. Any patient whose initial visit was for a heart attack was excluded from the study group.
Across all age groups included, the risk of myocardial infarction occurring after the initial hospital visit was markedly higher for those infected with HIV. Although traditional cardiovascular risk factors - such as elevated lipid levels, diabetes and hypertension - also were more common among the HIV-infected patients and did account for some increased risk, the increased risk for heart attack associated with HIV remained significant even when adjusted for those risk factors. Overall, the risk of heart attack was almost doubled in all those with HIV and was almost tripled among women.
"Followup studies are needed to better determine why myocardial infarction rates are higher in HIV patients, which risk factors drive this risk most, and how smoking - which we weren't able to completely evaluate in this study - affects this risk," Grinspoon says. "We also need to analyze the relationship of antiretroviral medications to cardiovascular risk. HIV medications save lives, and patients should continue taking them as prescribed; but we want physicians to be aware of these increased heart attack rates, watch risk factors carefully and appropriately target their treatment." Grinspoon is an associate professor of Medicine at Harvard Medical School.
Manuscript
"Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors Among Patients with HIV Disease"
Virginia A. Triant, M.D., M.P.H., Hang Lee, Ph.D., Colleen Hadigan, M.D., M.P.H., and Steven K. Grinspoon, M.D.
From the Massachusetts General Hospital (MGH) Program in Nutritional Metabolism (V.T., C.H. and S.G.), Brigham and Women's Hospital (BWH) and MGH Divisions of Infectious Diseases (V.T.) and the MGH Biostatistics Center (H.L.), Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114
".....neither the DAD study nor another large study that prospectively evaluated the effect of protease inhibitors on the development of AMI(10) included control groups for comparison in prospective analyses.....The rate of AMI per 1000 person years was higher among HIV patients in this study than in the DAD study, but our study included older patients as well and was from a U.S. population, with potentially different AMI rates and cardiovascular risk factors than the European-based population of the DAD study. Furthermore, the background rate among the entire reference population was similar to that seen in other large U.S. databases, including the Framingham Heart Study, suggesting that this study reliably captured AMI events across the two large hospitals for which we have data. Importantly, the relative risk of AMI in HIV patients was approximately two-fold increased over controls, and thus likely to be clinically significant.....These data suggest that cardiovascular disease will likely become an increasing problem for HIV-infected patients as the population lives longer and ages. Race was also significant in the overall model as an independent predictor of AMI.... A recent study showed a 14 percent prevalence rate of diabetes in HIV-infected men using ARV therapy compared to five percent in seronegative men.(3) Our data confirm and extend this data, showing an approximate two-fold increase in diabetes rates in HIV-infected patients relative to non- HIV patients..... we could not investigate the contribution of antiretroviral drugs to myocardial infarction rates in HIV-infected patients because specific data on duration of ARV use were not available through the database. Rather, the primary purpose of the study was to investigate myocardial infarction rates and major cardiac risk factors among patients with HIV disease in comparison to a large non-HIV cohort, controlling for common cardiac risk factors, age, race and gender. Smoking data were assessed in a subanalysis among patients for whom this data was available, demonstrating that the higher AMI risk remained in HIV patients despite higher smoking rates..... In summary, our data from a large cohort of HIV-infected patients demonstrate increased AMI rates and cardiovascular risk factors compared to a non-HIV population, particularly among women. The AMI event rate was approximately two-fold elevated in the HIV cohort, and this difference was seen over multiple age ranges."
Abstract
Context: Metabolic changes and smoking are common among HIV patients and may confer increased cardiovascular risk.
Objective: To determine acute myocardial infarction (AMI) rates and cardiovascular risk factors in HIV compared to non-HIV patients in two tertiary care hospitals.
Design, Setting and Participants: We conducted a health-care system-based cohort study using a large data registry with 3,851 HIV and 1,044,589 non-HIV patients. AMI rates were determined among patients receiving longitudinal care between October 1, 1996 and June 30, 2004.
Main Outcome Measures: The primary outcome was myocardial infarction, identified by International Classification of Diseases coding criteria.
Results: AMI was identified in 189 HIV and in 26,142 non-HIV patients. AMI rates per 1000 person years were increased in HIV versus non-HIV patients (11.13 [95% CI 9.58- 12.68] vs. 6.98 [95% CI 6.89-7.06]). The HIV cohort had significantly higher proportions of hypertension (21.2 vs. 15.9 percent), diabetes (11.5 vs. 6.6 percent), and dyslipidemia (23.3 vs. 17.6 percent) than the non-HIV cohort (P<0.0001 for each comparison). The difference in AMI rates between HIV and non-HIV patients was significant, with a relative risk (RR) of 1.75 (95% CI 1.51-2.02; P<0.0001), adjusting for age, gender, race, hypertension, diabetes, and dyslipidemia. In gender-stratified models, the unadjusted AMI rates per 1000 person years were higher for HIV patients among women (12.71 vs. 4.88 for HIV compared to non-HIV women) but not among men (10.48 vs. 11.44 for HIV compared to non-HIV men). The relative risks were 2.98 (95% CI 2.33-3.75; P<0.0001) for women and 1.40 (95% CI 1.16-1.67; P=0.0003) for men, adjusting for age, gender, race, hypertension, diabetes, and dyslipidemia. A limitation of this database is that it contains incomplete data on smoking. Smoking could not be included in the overall regression model and some of the increased risk may be accounted for by differences in smoking rates.
Conclusions: AMI rates and cardiovascular risk factors were increased in HIV compared to non-HIV patients, particularly among women. Cardiac risk modification strategies are important for the long-term care of HIV patients.
Introduction
Metabolic abnormalities are known to occur frequently in human immunodeficiency virus (HIV) infected individuals and include dyslipidemia,(1) insulin resistance and diabetes,(2, 3) endothelial dysfunction(4, 5) and altered fat distribution.(1, 6-9) Myocardial infarction rates have been shown to be increased in association with protease inhibitor (PI) use (10) and duration of antiretroviral (ARV) therapy in studies limited to HIV patients.(11) However, it remains unclear as to whether HIV infected patients demonstrate increased rates of acute myocardial infarction (AMI) compared to non-HIV infected patients.
In the current study, we compared myocardial infarction rates among HIV-infected patients receiving longitudinal care in two large U.S. hospitals to background longitudinal rates among the entire population not diagnosed with HIV infection at these hospitals. We determined the relative risk (RR) for AMI associated with HIV, adjusting for age, race, gender and common cardiac risk factors, including hypertension, diabetes and dyslipidemia.
Methods
Study Design
We conducted a cohort study of 3,851 patients diagnosed with HIV disease and
1,044,589 patients not diagnosed with HIV disease. All patients in the analysis, HIV and non-HIV infected, presented on at least two occasions to one of two Boston health care facilities, Brigham and Womenfs Hospital (BWH) or Massachusetts General Hospital (MGH), comprising the Partners HealthCare System. The non-HIV cohort represents all patients not diagnosed with HIV disease who presented to BWH or MGH on at least two occasions. Cohorts were identified by querying a data registry of all billed hospital encounters, the Research Patient Data Registry (RPDR). The primary outcome was AMI, defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). IRB approval was obtained from the Partners Human Research Committee.
Eligibility Criteria
Patients with at least two encounters of any kind between October 1, 1996 and
June 30, 2004 were included. The start date of 1996 marks the full integration of BWH and MGH inpatient and outpatient data into the RPDR. Patients under the age of 18 and over the age of 84 were excluded based on inadequate representation in the cohort of HIV-infected patients. Patients with only one encounter and patients for whom an AMI event was the first encounter were excluded from the analysis, as duration of follow-up in the cohort could not be calculated. In cases of recurrent AMIs, patients were only counted once in the analysis.
Data Source
Patient data were obtained from the RPDR, a centralized clinical data registry
containing comprehensive demographic and clinical information for more than 2.1 million patients seen at BWH since 1996 or MGH since 1992. Data are derived from several sources, including hospital billing systems and a clinical data repository. The data warehouse has an online query tool which can be used to return a set of patients that match complex search criteria. Patient identifiers are encrypted throughout the database. Information on demographics, encounters, diagnoses, and laboratories is accessible.
Ascertainment of Outcomes
The primary endpoint was AMI. Cases were determined by International
Classification of Diseases (ICD) coding and included all patients with ICD-9-CM code 410 (acute myocardial infarction) and all subtypes as entered in the RPDR. The diagnosis of HIV was based on ICD-9-CM coding of either 042 (human immunodeficiency virus disease) or 043 (human immunodeficiency virus infection causing other specified conditions).
Information was also collected on traditional cardiac risk factors, including hypertension, diabetes, and dyslipidemia, using ICD-9-CM codes 401 (essential
hypertension), 250 (diabetes mellitus), and 272 (disorders of lipoid metabolism) and all subtypes, respectively. Information on the use of PIs, nucleoside reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) was determined through medication records when available. Available smoking data were recorded.
Statistical Analysis
AMI incidence rates per 1000 person years contributed during the observation
period were determined for the HIV and non-HIV cohorts. Duration of individual patient follow-up was determined from date of first encounter to date of last encounter or date of event. Data on exact duration of follow-up was available for 1,048,440 patients and was approximated to the relevant boundary of the follow-up period (October 1, 1996 or June 30, 2004) for the remaining patients if the initiation date was prior to the beginning of the study time period or the end date was after the end of the study time period. Age-specific rates were determined, and 95 percent confidence intervals (CIs) were obtained using the method of large sample approximation. Age categories were constructed with the following breakdown: 18 to 34, 35 to 44, 45 to 54, 55 to 64, 65 to 74, and 75 to 84 years.
Multivariate Poisson regression models were used to assess the incidence rate ratio, the measure of relative risk, for AMI by HIV status in a combined model of all patients, adjusting for age, gender, race, hypertension, diabetes and dyslipidemia. The multivariate model was constructed a priori using relevant demographic and clinical covariates for which information was available. Covariate effects were simultaneously determined. A sensitivity analysis was also performed, investigating the effects of individual covariates in Poisson regression modeling. Stratified analyses were then performed to examine these effects among the HIV and non-HIV cohorts, and among women and men.
To assess validity of the models, we first assessed the Poisson model and did not find evidence of overdispersion. We also performed a Cox proportional hazards regression analysis. The hazard ratios were almost identical to the incidence rate ratios from Poisson regression analysis and the data satisfied the Cox proportional hazard assumptions. In addition, we employed the Generalized Estimating Equations (GEE) estimation to obtain a robust standard error of the RR allowing for the potential of misspecification of the model. Poisson regression analysis incidence rate ratios and person time incidence rate estimates are reported in this manuscript.
AMI diagnoses were validated in a subset of patient medical records (100 HIV
and 100 non-HIV patients randomly selected from the entire study population).
Validation criteria included one or more of the following in the medical record: elevation of cardiac enzymes above the normal laboratory limit, CABG or cardiac catheterization with angioplasty, or clear documentation of clinical myocardial infarction event in medical record. Using these criteria, AMI rates could be validated in up to approximately 50 percent for both the HIV and non-HIV groups. We conducted a sensitivity analysis to reflect the possibility of misclassification bias. In this analysis, we randomly discarded 50 percent of the observed AMI cases from both the HIV and non-HIV groups (to reflect the estimated maximal boundary of error) and fit a proportional hazards model to each dataset. The analysis was repeated 20 times.
To evaluate the effect of smoking, we compared the proportion of AMI between
HIV and non-HIV patients by a stratified Mantel-Haenszel analysis where smoking strata were never versus ever (current or past) smoking. SAS statistical software was used for all analyses.
Results
Demographic and Clinical Characteristics
The HIV cohort included 3,851 patients. Of these, 1,172 (30.4 percent) were women and 2,679 (69.6 percent) were men. In the HIV cohort, there were 16,983 person years (PYs) contributed during the study period, of which 5,430 PYs were contributed by women. The mean time of observation for HIV patients was 4.5 years. Percentages using major classes of antiretroviral drugs are shown in Table 1.
The non-HIV cohort included 1,044,589 patients, of whom 617,396 (59.1 percent)
were women and 427,189 (40.9 percent) were men. Total patients in the non-HIV cohort contributed 3,747,329 PYs during the observation period. Of these, women contributed 2,217,127 PYs. The mean time of observation for non-HIV patients was 3.7 years. The mean and median ages were similar between the groups. Median age was 38 (interquartile range 12) for HIV versus 39 (interquartile range 26) for non-HIV patients (Table 1). However, within the HIV group men in younger age categories contributed a greater percentage to the person time denominator relative to the non-HIV group than did younger women.
The HIV cohort had a higher proportion of African-American and Hispanic
patients than the non-HIV cohort, with patients categorized as African American
comprising 23.5 percent versus 6.7 percent, and patients categorized as Hispanic comprising 12.7 percent versus 7.1 percent of the HIV versus non-HIV cohort, respectively. Distribution of race according to gender is shown in Table 1.
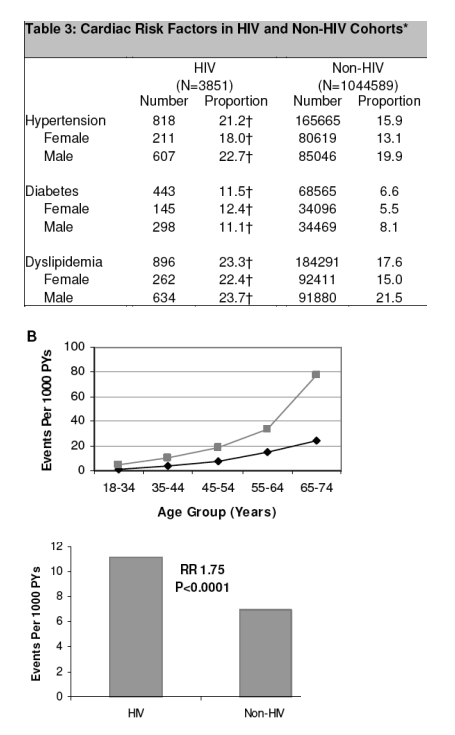
As shown in Table 3, the HIV cohort had significantly higher proportions of
hypertension (21.2 vs. 15.9 percent), diabetes (11.5 vs. 6.6 percent), and dyslipidemia (23.3 vs. 17.6 percent) than the non-HIV cohort (P<0.0001 for each comparison). Increased percentages of these risk factors were also seen in the gender-stratified comparisons of men and women (Table 3).
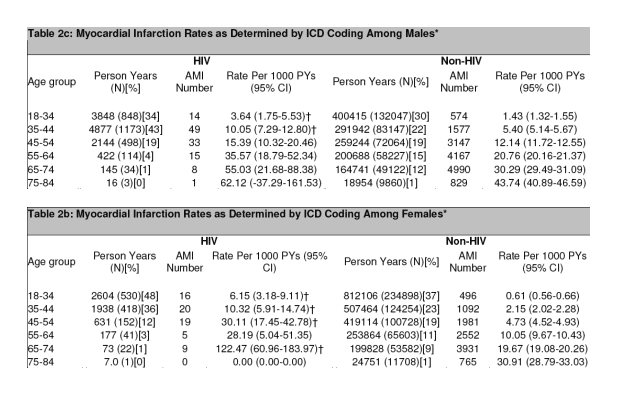
Myocardial Infarction Rates
AMI was identified in 189 patients in the HIV cohort and in 26,142 patients in the
non-HIV cohort. Across all age groups, the rate of AMI was consistently higher for patients in the HIV cohort compared to the non-HIV cohort (Table 2a and Figure 1B). The rates of AMI were 11.13 per 1000 PYs for HIV (95% CI 9.58-12.68) and 6.98 per 1000 PYs for non-HIV (95% CI 6.89-7.06) (Figure 1A). The unadjusted relative risk was 1.53 (95% CI 1.32-1.75; P<0.0001). This increase in AMI rates in HIV compared to non-HIV patients remained significant when the data were adjusted for age, gender, race, hypertension, diabetes, and dyslipidemia, with a relative risk of 1.75 (95% CI 1.51-2.02; P<0.0001) in a combined regression model. Smoking was not included in the model due to limited data. In addition to HIV disease, risk factors for AMI included male gender (RR 1.72; 95% CI 1.68-1.77; P<0.0001), older age (RR 17.24 for ages 75-84
vs. 18-34; 95% CI 15.87-18.52; P<0.0001), African-American race (RR 1.44 compared to Caucasians; 95% CI 1.38-1.50; P<0.0001), diabetes (RR 1.98; 95% CI 1.93-2.04; P<0.0001), hypertension (RR 1.62; 95% CI 1.57-1.66; P<0.0001) and dyslipidemia (RR 3.03; 95% CI 2.93-3.13; P<0.0001) in a combined model.
We also assessed the effects of individual covariates in the model. With the
addition of age and gender into the model, the RR was 2.45 (P<0.0001). With race added to age and gender, the RR was 2.09 (P<0.0001). Cardiac risk factors were next tested individually in the model after adjustment for demographic covariates. The RR for AMI based on HIV status was 2.01 (P<0.0001) in a model adjusted for demographic covariates and hypertension. The RR for AMI was 1.89 (P<0.0001) adjusting for demographic covariates and diabetes and also 1.89 (P<0.0001) adjusting for demographic covariates and dyslipidemia. The inclusion of hypertension attenuated the RR by 4 percent, whereas the inclusion of either diabetes or dyslipidemia attenuated the RR by 10 percent. Among the cardiac risk covariates tested individually in the model, the RR for AMI was largest
for dyslipidemia (RR 4.5), followed by diabetes (RR 3.3) and then hypertension (RR 3.0) (all P values <0.0001). The same analysis was performed with Cox modeling with similar results.
The difference in AMI rates was more pronounced in women. In gender-stratified
models the unadjusted rates per 1000 PYs were higher for the HIV group in the
comparison of women (12.71 vs. 4.88 for HIV compared to non-HIV women) but not in men (10.48 vs. 11.44 for HIV compared to non-HIV men) (Tables 2b and 2c for age breakdown). The relative risks of AMI, comparing HIV to non-HIV, were 2.98 (95% CI 2.33-3.75; P<0.0001) for women and 1.40 (95% CI 1.16-1.67; P=0.0003) for men, adjusting for age, race, hypertension, diabetes, and dyslipidemia.
Individual covariates were also assessed in gender stratified models. Among men, the relative risk increased to 1.73 (95% CI 1.40-2.06; P<0.0001) with adjustment for age alone, and was then attenuated to 1.44 (95% CI 1.20-1.72, P<0.0001) with adjustment for race and cardiovascular risk factors (hypertension, diabetes, dyslipidemia). Among women, the relative risk increased to 5.74 (95% CI 4.49-7.22; P<0.0001) with adjustment for age alone, and was then attenuated to 3.12 (95% CI 2.44-3.93, P<0.0001) with adjustment for race and cardiovascular risk factors (hypertension, diabetes, dyslipidemia).
Risk Factors for AMI in Stratified Models of HIV and Non-HIV
We stratified on the basis of HIV status in order to determine risk factors for AMI
within the HIV group and the non-HIV group. Among the HIV-infected patients,
dyslipidemia was the cardiac risk factor most significantly associated with AMI,
controlling for age, race, gender and other cardiac risk factors (RR 3.65; 95% CI 2.59-5.19; P< 0.0001). Hypertension (RR 1.23; 95% CI 0.90-1.68; P=0.20) and diabetes (RR 1.33; 95% CI 0.95-1.85; P=0.09) were also associated with AMI within the HIV group, but these associations did not reach statistical significance. African-American race was a significant predictor of AMI among HIV patients, with a relative risk of 1.43 (95% CI 1.01-2.00; P=0.04). Among the non-HIV patients, dyslipidemia was also the cardiac risk factor most significantly associated with AMI, controlling for age, race, gender and other cardiac risk factors (RR 3.02; 95% CI 2.92-3.12; P< 0.0001). Hypertension (RR 1.62;
95% CI 1.58-1.67; P<0.0001) and diabetes (RR 1.99; 95% CI 1.93-2.04; P<0.0001) were significantly associated with AMI in the non-HIV group.
Effect of Smoking on the Association of HIV and AMI
Smoking data were available in 854, or 22 percent of HIV patients, of whom 57
percent were current or prior smokers and 38 percent were current smokers. Among women with HIV, approximately 51 percent were current or prior smokers and 33 percent were current smokers. Among men with HIV, approximately 62 percent were current or prior smokers and 41 percent were current smokers. Among the non-HIV group, smoking data were available in 111,296, or 11 percent of patients of whom 40 percent were current or prior smokers and 18 percent were current smokers. For non-HIV women, approximately 37 percent were current or prior smokers and 16 percent were current smokers. For non-HIV men, approximately 48 percent were current or prior smokers and 22 percent were current smokers.
Among non-smokers, the rates of AMI in HIV versus non-HIV were 7.08 versus
2.64 percent (P<0.0001). Among smokers, the rates of AMI in HIV versus non-HIV were 7.80 versus 6.15 percent (P=0.13). The common odds ratio estimate for risk of AMI (HIV versus non-HIV) by Mantel-Haenszel analysis was 1.7 controlling for smoking (95% CI 1.3-2.2, HIV vs. non-HIV).
Validation Subset and Sensitivity Analysis
We conducted a sensitivity analysis to reflect the possibility of misclassification
bias as outlined in the methods. The hazard ratio estimate over the 20 iterations resulted in an empirical mean hazard ratio of 1.69 (standard deviation
DISCUSSION
Prior studies examining myocardial infarction rates among HIV patients have
been limited by several factors, including the absence of a reference population. The DAD study provided important information regarding the duration-related association of antiretroviral therapy with AMI rates among HIV patients.(11) However, neither the DAD study nor another large study that prospectively evaluated the effect of protease inhibitors on the development of AMI(10) included control groups for comparison in prospective analyses. Our study included comparative data and specifically assessed whether AMI rates are elevated in HIV patients compared to a non-HIV reference population. In contrast to the DAD study, the purpose of this study was not to determine the relationship of AMI rates to ARV use, as detailed information on the longitudinal use of ARVs could not be captured from the database, but rather to compare myocardial
infarction rates between HIV and non-HIV patients, followed at two large U.S. hospitals.
The rate of AMI per 1000 person years was higher among HIV patients in this study than in the DAD study, but our study included older patients as well and was from a U.S. population, with potentially different AMI rates and cardiovascular risk factors than the European-based population of the DAD study. Furthermore, the background rate among the entire reference population was similar to that seen in other large U.S. databases, including the Framingham Heart Study, suggesting that this study reliably captured AMI events across the two large hospitals for which we have data. Importantly, the relative risk of AMI in HIV patients was approximately two-fold increased over controls, and thus likely to be clinically significant.
Several other studies that have included reference populations have used endpoints whose broad or composite nature may have decreased specificity. Two large studies that determined cases based on insurance claims from Medicaid and health maintenance organization data used coronary heart disease (CHD) as an endpoint.(12, 13)
When evaluated for AMI instead of CHD, one study showed only a trend towards higher rates in the HIV group.(13) A second study demonstrated increased CHD incidence among younger patients ages 25 to 34 using one of five ICD codes for ischemic heart disease.(12) A study of patients in the Veterans Affairs system, from an earlier time period - 1993 to 2001 - used five composite endpoints and showed decreased hospitalization rates, but did not distinguish cardiovascular from cerebrovascular disease or AMI from CHD.(14) In contrast, our data using the specific ICD code for AMI as opposed to CHD, demonstrate increased AMI rates for all age groups relative to the non-HIV cohort. A study based on data from the Womenfs Health Initiative showed strong agreement between hospital discharge code of myocardial infarction and validation of events by study physicians for the diagnosis of myocardial infarction.(15)
There was no difference in the overall unadjusted AMI rates in the comparison of
HIV-infected to -uninfected men. Among men the relative risk became highly significant in a gender-stratified model after adjusting for age. With adjustment for all demographic and clinical variables, the RR of AMI remained highly significant in the comparison of male HIV versus non-HIV groups. As expected, the RR was attenuated by approximately 17 percent when adjusting for hypertension, diabetes and dyslipidemia. Both the unadjusted event rates and the adjusted RR were higher in the comparison of female HIV to non-HIV groups. In the gender-stratified model for women, adjustment for age increased the relative risk in a pattern similar to that of men. However, adjustment for hypertension, diabetes and dyslipidemia attenuated the RR by 46 percent among women.
Prior studies have shown that women demonstrate increased cardiovascular disease risk indices associated with fat redistribution,(16) but only limited information is available regarding the risk of cardiac events in HIV-infected women. Two major studies excluded women due to a small number of outcomes.(10, 13) Data from a study based on California Medicaid claims suggested an increased AMI incidence among HIV-infected women ages 25 to 34.(12) In this study, we conducted both combined regression and
gender-stratified models. In both male and female models including age, race and common cardiac risk factors, HIV status was a significant predictor of AMI, but the relative risk of AMI was higher in women (RR 2.98) than in men (RR 1.40), possibly because of altered inflammatory marker profiles,(16) significant changes in body composition in this group, and relative shift from gynoid to android fat distribution,(7) or other factors. Indeed our data suggest that the rates of some common cardiac risk factors, such as diabetes, may be relatively greater in women.
Age and race were significant independent risk factors for AMI. The risk of AMI
increased with age in both the HIV and non-HIV groups, and increased AMI rates were seen for all ages in the comparison of HIV and non-HIV patients. These data suggest that cardiovascular disease will likely become an increasing problem for HIV-infected patients as the population lives longer and ages. Race was also significant in the overall model as an independent predictor of AMI. AMI risk was significantly increased among African-American relative to Caucasians and in Hispanics relative to Caucasians. Among HIV patients, African American race was significant as a predictor of AMI. Prior data suggest that there is an association between race and cardiovascular disease, with higher median rates of cardiovascular events (defined as AMI, angina, CHD, or stroke) compared to the national average for African-American women and lower rates for Hispanic women and men and African-American men.(17) Racial differences between the HIV and non-HIV groups were accounted for in Poisson regression modeling, which showed a significant relative risk of 1.75 for AMI in the HIV group relative to the non- HIV group.
Metabolic changes are common in HIV-infected patients and likely play a role in
the development of atherosclerosis, possibly in concert with other cardiac risk factors. Our study demonstrates increased rates of traditional comorbidities, including hypertension, diabetes, and dyslipidemia, seen at rates of 21.2, 11.5 and 23.3 per 100 persons in the HIV cohort, respectively. An increased rate of dyslipidemia has been well documented in smaller studies of HIV-infected populations and may relate to effects of HIV as well as antiretroviral therapy.(18, 19) Among the Swiss HIV cohort, classification of hypercholesterolemia was seen in approximately one third of patients on protease inhibitors.(8) The dyslipidemia rate of 23.3 per 100 persons seen in our study was determined from a large HIV cohort not selected for PI use, providing further evidence for the widespread prevalence of dyslipidemia in this population. Dyslipidemia was an
independent risk factor for AMI in separate models of HIV and non-HIV and in the fully adjusted model of the combined groups (RR 3.03; 95% CI 2.93-3.13; P<0.0001), controlling for age, gender, race, hypertension and diabetes.
In this study, we also demonstrate increased rates of diabetes and hypertension in the HIV compared with the non-HIV cohort. Increased risk factors were seen in both the male and female comparisons, in gender stratified analyses. A recent study showed a 14 percent prevalence rate of diabetes in HIV-infected men using ARV therapy compared to five percent in seronegative men.(3) Our data confirm and extend this data, showing an approximate two-fold increase in diabetes rates in HIV-infected patients relative to non- HIV patients. Only limited data are available regarding hypertension rates. Another group demonstrated elevated rates of hypertension in a small study of HIV-infected patients.(20) Our data add further emphasis to the potential role played by this and other cardiac risk factors. Both diabetes and hypertension were independent risk factors for AMI in the fully adjusted model of the combined groups (RR 1.98; 95% CI 1.93-2.04; P<0.0001 for diabetes and RR 1.62; 95% CI 1.57-1.66; P<0.0001 for hypertension). Sensitivity analyses suggested that diabetes and dyslipidemia, more than hypertension, contributed to the increase in AMI rates among the HIV group.
Relatively little is known about the risk of smoking and cardiovascular disease in
HIV patients. Among non-HIV groups, numerous cohort studies demonstrate increased rates of CHD for smokers, with relative risks among current smokers as opposed to never-smokers ranging from 1.3 to 3.5.(21) The DAD study suggested that smoking was an independent risk for AMI in HIV patients, but did not perform a comparison to control patients, controlling for smoking use. The estimated common odds ratio obtained from the analysis by smoking status in patients for whom smoking data were available in our study was comparable to that obtained using the multivariate Poisson regression model approach. However, due to database constraints, smoking data were available in only a
subset of the patients, and thus could not be added to the overall model. It is possible that increased smoking rates in the HIV group contribute to the increased rate of AMI in the HIV group. Other factors not measured, including inflammation, (22, 23) direct effects of the virus, or antiretroviral therapy, (4, 24, 25) may also contribute to the increased risk of AMI demonstrated in the HIV group.
This study investigates AMI events in both HIV and non-HIV patients followed
longitudinally at two large U.S. hospitals. Furthermore, adequate women were studied to develop gender-stratified models, showing that HIV status was a relatively greater predictor of AMI among women than men. Background comparison rates for AMIs in the entire cohort of over one million are similar to those reported in other large outpatient databases,(26) suggesting that the database reliably captured AMI events. The relatively long and similar duration of follow-up between HIV and non-HIV groups and exclusion of all patients with a single isolated encounter, or initial encounter being an AMI, ensures that rates were determined in patients receiving longitudinal follow-up in the system,
rather than patients acutely referred in for AMI assessment and treatment.
The study was limited by the constraints of the database. Overall rates of ARV
use were similar to other large cohorts, but we could not investigate the contribution of antiretroviral drugs to myocardial infarction rates in HIV-infected patients because specific data on duration of ARV use were not available through the database. Rather, the primary purpose of the study was to investigate myocardial infarction rates and major cardiac risk factors among patients with HIV disease in comparison to a large non-HIV cohort, controlling for common cardiac risk factors, age, race and gender. Smoking data were assessed in a subanalysis among patients for whom this data was available, demonstrating that the higher AMI risk remained in HIV patients despite higher smoking rates. However, due to lack of uniformly available data, this information was not able to be included in the overall regression model. Use of ICD coding to define AMI is standard in large epidemiological studies but is subject to misclassification bias. We performed a sensitivity analyses based on data obtained from a validation subset to determine the effect of possible miclassification bias. The hazard ratio in the sensitivity analysis, testing
a 50 percent maximum misclassification rate, was 1.69, compared to 1.75. In addition, hypertension and dyslipidemia could only be included as dichotomous rather than quantitative traits, as we were limited to the use of ICD codes in the study, and this may have limited the strength of the covariate analysis. Thus, it is not possible to know whether the differences between HIV and non-HIV after adjustment are due to HIV per se or the limited nature of covariate information in the database.
In summary, our data from a large cohort of HIV-infected patients demonstrate increased AMI rates and cardiovascular risk factors compared to a non-HIV population, particularly among women. The AMI event rate was approximately two-fold elevated in the HIV cohort, and this difference was seen over multiple age ranges. Relative myocardial infarction rates were greater among women, in comparison to the non-HIV cohort, and this gender-specific difference may account in part for the overall differences we observed. Determining the mechanisms of increased cardiovascular disease and cardiac risk factor rates in HIV-infected women is an important area of future research. Cardiac risk modification strategies are also particularly needed and will be an important component of the long-term care of this population.
|
|
| |
| |
|
|
|