| |
Class of Antiretroviral Drugs and the Risk of Myocardial Infarction, The DAD Study Group
|
| |
| |
NEJM April 26, 2007
The members of the DAD Study Group are as follows: Steering committee: J.D. Lundgren (chair), R. Weber, A. d'Arminio Monforte, W.M. El-Sadr, P. Reiss, F. Dabis, L. Morfeldt, S. De Wit, C. Pradier, G. Calvo, M. Law, O. Kirk, A.N. Phillips, S. Collins, E. Loeliger, R. Tressler, and I. Weller; Central coordination: N. Friis-Moller, S.W. Worm, C.A. Sabin, A. Sjol (verification of primary end point), J.D. Lundgren; Data management: A. Sawitz (coordinator), M. Rickenbach, P. Pezzotti, E. Krum, L. Gras, E. Balestre, A. Sundstrom, B. Poll, E. Fontas, F. Torres, K. Petoumenos, J. Kjor.
Note from Jules: again, in an Editorial in this NEJM James Stein says the findings reported from the DAD study are "unclear" with overlapping confidence intervals, lower or similar rates of risk perhaps as tradional cofactors such as diabetes and smoking. In addition, the DAD study says increased risk associated with PIs are associated with increased lipids yet the recent ACTG 5142 metabolics substudy presented at CROI 2007 found efavrienz associated with greater risk for lipoatrophy and similar lipids profile compared with Kaletra. The SMART study found ART interruptions associated with cardiovascular risk yet it does not appear that the DAD study consider interruptions in their analysis. In addition, Steve Grinspoon published a study this week in the Journal of Clinical Endocrinology finding that HIV+ individuals had almost 2 fold greater risk for heart attack than HIV-negative control group and HIV and tradional risk factors were important. Quite a mix of considerations.
Abstract
Background We have previously demonstrated an association between combination antiretroviral therapy and the risk of myocardial infarction. It is not clear whether this association differs according to the class of antiretroviral drugs. We conducted a study to investigate the association of cumulative exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors with the risk of myocardial infarction.
Methods We analyzed data collected through February 2005 from our prospective observational study of 23,437 patients infected with the human immunodeficiency virus. The incidence rates of myocardial infarction during the follow-up period were calculated, and the associations between myocardial infarction and exposure to protease inhibitors or nonnucleoside reverse-transcriptase inhibitors were determined.
Results Three hundred forty-five patients had a myocardial infarction during 94,469 person-years of observation. The incidence of myocardial infarction increased from 1.53 per 1000 person-years in those not exposed to protease inhibitors to 6.01 per 1000 person-years in those exposed to protease inhibitors for more than 6 years. After adjustment for exposure to the other drug class and established cardiovascular risk factors (excluding lipid levels), the relative rate of myocardial infarction per year of protease-inhibitor exposure was 1.16 (95% confidence interval [CI], 1.10 to 1.23), whereas the relative rate per year of exposure to nonnucleoside reverse-transcriptase inhibitors was 1.05 (95% CI, 0.98 to 1.13). Adjustment for serum lipid levels further reduced the effect of exposure to each drug class to 1.10 (95% CI, 1.04 to 1.18) and 1.00 (95% CI, 0.93 to 1.09), respectively.
Conclusions Increased exposure to protease inhibitors is associated with an increased risk of myocardial infarction, which is partly explained by dyslipidemia. We found no evidence of such an association for nonnucleoside reverse-transcriptase inhibitors; however, the number of person-years of observation for exposure to this class of drug was less than that for exposure to protease inhibitors.
Combination antiretroviral therapy has had a dramatic effect in reducing morbidity and mortality associated with human immunodeficiency virus type 1 (HIV-1) infection.1,2,3,4 However, concern has been raised regarding the effect of such therapy on the risk of coronary heart disease. Previous findings from the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study indicated that the incidence of myocardial infarction increased with longer exposure to combination antiretroviral therapy.5
A central question is whether this observed risk is attributable to all antiretroviral drugs or only to specific drugs. Although previous studies have reported a relationship between protease-inhibitor use and cardiovascular disease,6,7,8 there are few data on the risk associated with nonnucleoside reverse-transcriptase inhibitors. Assessment of the role of specific drug classes is complicated by the fact that patients often switch components of their treatment because of the availability of newer drugs, the occurrence of adverse events, or failure of their regimens.9,10,11
There have been 3 years of follow-up of patients in the DAD study since the initial report.5 The additional data have made it possible to investigate the independent relationships between the risk of myocardial infarction and exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors.
Results
Patient Characteristics and Follow-up
The DAD study cohort included 23,437 patients (Table 1). The median age at enrollment was 39 years (interquartile range, 34 to 45), and 24.1% of the patients were female. Among the 61.0% of patients for whom information on race or ethnic background (ascertained by different methods in different centers) was available, 77.9% were white, 16.9% were black, 3.3% were Hispanic, 1.9% were Asian, and 0.1% (nine patients) were classified as "other." The median nadir CD4+ lymphocyte count before enrollment was 200 cells per cubic millimeter (range, 1 to 2580), and 26.4% of patients had received a clinical diagnosis of the acquired immunodeficiency syndrome (AIDS). At enrollment, 60.8% of participants were current or former smokers, 3.1% had diabetes, 14.4% had hypertension, and 42.0% had dyslipidemia.
The total follow-up in this analysis was 94,469 person-years, with a median of 4.5 years per patient. By the end of follow-up, 1518 patients (6.5%) had died. The average annual rate of loss to follow-up, excluding deaths, was less than 3%.
Exposure to Antiretroviral Therapy
By the end of follow-up, 93.6% of the patients had been exposed to some form of antiretroviral therapy, totaling 150,775 person-years of exposure, with a median exposure of 6.9 years (Table 1). Of all the patients, 79.4% had been exposed to protease inhibitors for a median of 4.0 years, and 63.7% had been exposed to nonnucleoside reverse-transcriptase inhibitors for a median of 2.6 years. There was a total of 72,846 person-years of exposure to protease inhibitors (including 41,297 person-years of exposure to ritonavir-containing regimens) and 52,457 person-years of exposure to nonnucleoside reverse-transcriptase inhibitors.
Myocardial Infarction
Three hundred forty-five patients had a fatal or nonfatal myocardial infarction (incidence, 3.65 per 1000 person-years) (Table 1). Of these, 62.6% were definite, 22.6% were possible, and 14.8% were unclassifiable; 29.3% were fatal. By the time of the event, 90.4% of patients who had a myocardial infarction had been exposed to protease inhibitors (median exposure, 3.7 years), and 60.9% had been exposed to nonnucleoside reverse-transcriptase inhibitors (median exposure, 2.1 years).
Association of Antiretroviral Therapy with the Risk of Myocardial Infarction
We confirmed our previous observation that increased exposure to combination antiretroviral therapy was associated with an increased risk of myocardial infarction (adjusted relative rate, 1.16 per year of exposure; 95% confidence interval [CI], 1.09 to 1.23) (Figure 1). The therapy-attributed relative rate did not differ significantly between men (relative rate, 1.13) and women (relative rate, 1.36; P value for interaction=0.40) or between older patients (>45 years of age for men, and >55 years of age for women; relative rate, 1.15) and younger patients (relative rate, 1.16; P value for interaction=0.19).
The incidence of myocardial infarction increased with increasing length of exposure to either class of drugs (Figure 2A and Table 2, unadjusted model); for example, the incidence increased from 1.53 per 1000 person-years among those not exposed to protease inhibitors to 6.01 per 1000 person-years among those exposed to protease inhibitors for more than 6 years. However, because many patients had been exposed to both drug classes, the unadjusted event rates are not independent. After adjustment for exposure to the other drug class, calendar year, and other known risk factors for myocardial infarction (excluding those that have been reported to have an association with antiretroviral-drug therapy), the relative rate per year of exposure to protease inhibitors was 1.16 (95% CI, 1.10 to 1.23), whereas for nonnucleoside reverse-transcriptase inhibitors it was 1.05 (95% CI, 0.98 to 1.13; Figure 2B and Table 2, adjusted model 1). These associations were confirmed when patients exposed to the other drug class were excluded (Figure 3). The adjusted relative rate per year of exposure to protease inhibitors for this subgroup of patients was 1.15 (95% CI, 1.06 to 1.25). The adjusted relative rate per year of exposure to nonnucleoside reverse-transcriptase inhibitors was 0.94 (95% CI, 0.74 to 1.19).
Figure 3. Risk of Myocardial Infarction According to Exposure to Protease Inhibitors and Nonnucleoside Reverse-Transcriptase Inhibitors among Patients Unexposed to the Other Drug Class.
Cumulative exposure was categorized as no exposure, less than 1 year, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, and more than 6 years for protease inhibitors and as no exposure, less than 1 year, 1 to 2 years, 2 to 3 years, 3 to 4 years, and more than 4 years for nonnucleoside reverse-transcriptase inhibitors. Panel A shows the incidence of myocardial infarction according to the cumulative exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors among patients who had not been exposed to the other drug class. The crude incidence rates among patients exposed to nonnucleoside analogues were considerably lower in this analysis than in the analysis including all subjects (Fig. 2A), although the confidence intervals were much wider because of the smaller number of subjects included in these analyses. Panel B shows the unadjusted relative rates of myocardial infarction according to exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors among patients who had not been exposed to the other drug class. After exposures were fitted as continuous variables, the adjusted relative rate per year of exposure to protease inhibitors was 1.15 (95% CI, 1.06 to 1.25), and per year of exposure to nonnucleoside reverse-transcriptase inhibitors was 0.94 (95% CI, 0.74 to 1.19). These are two separate analyses, which were both adjusted for age, sex, family history of coronary heart disease, body-mass index, smoking status, history of cardiovascular disease, transmission group, race or ethnic group, and calendar year. The I bars denote the 95% CIs.
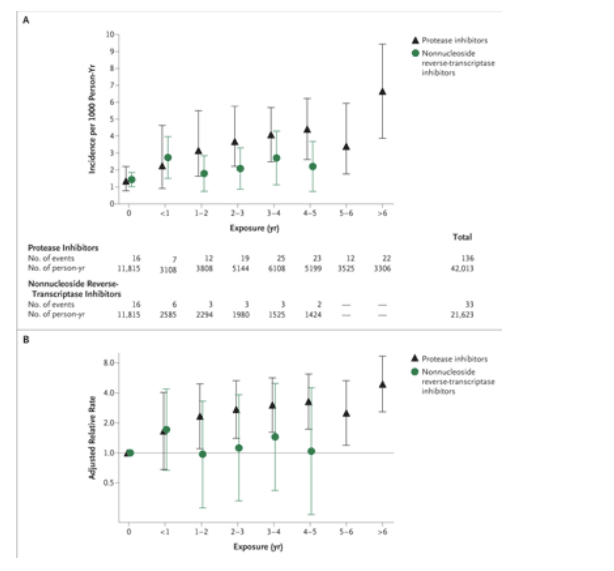
The crude incidence rate of myocardial infarction was increased among patients who received drugs of both classes at the same time (97 events in 16,805 person-years; incidence, 5.77 per 1000 person-years) as compared with the entire cohort (incidence, 3.65 per 1000 person-years). However, this difference was explained by the longer exposure to protease inhibitors of patients receiving drugs of both classes (relative rate for exposure to both drug classes after adjustment for duration of exposure to each class, 1.03 per year).
Controlling for exposure to nucleoside reverse-transcriptase inhibitors as a class reduced the strength of the associations for protease inhibitors (relative rate, 1.11; 95% CI, 1.04 to 1.18) and for nonnucleoside reverse-transcriptase inhibitors (relative rate, 1.02; 95% CI, 0.95 to 1.10). There were no major differences between the two drug classes in exposure to individual components of the nucleoside reverse-transcriptase inhibitor backbone.
Increased total cholesterol and reduced HDL cholesterol levels, increased triglyceride levels, and a diagnosis of hypertension or diabetes are risk factors for coronary heart disease that have been reported to be associated with antiretroviral-drug therapy. We confirmed that in our cohort, these factors were all associated with an increased risk of myocardial infarction in unadjusted analyses (Table 2, unadjusted model). Introducing these factors into adjusted model 1 in Table 2 as time-updated covariates decreased the association between exposure to protease inhibitors and the risk of myocardial infarction (Table 2, adjusted model 2). A model that included the lipids only (without diabetes mellitus and hypertension) reduced the association with protease inhibitors to a value similar to that in adjusted model 2.
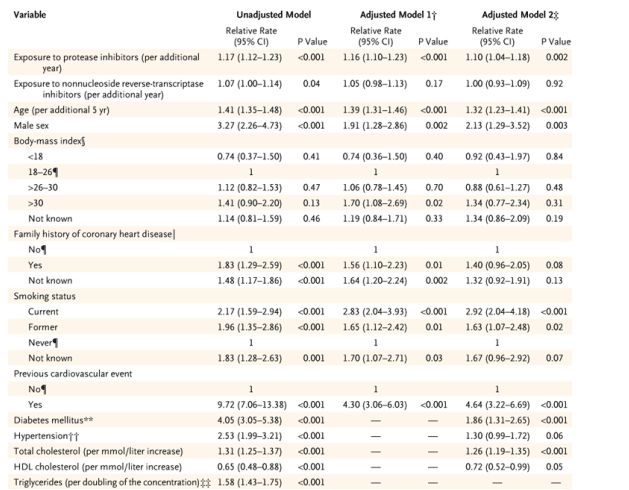
Table 2. Relationship between Exposure to Protease Inhibitors, Nonnucleoside Reverse-Transcriptase Inhibitors, and Other Cardiovascular Risk Factors and the Rate of Myocardial Infarction.
The type is small in this picture of the table but after adjusting (model 2): "Exposure to protease inhibitors (per additional year)" was associated with 1.10 (1.04-1.18) Relative Rate 95% CI, p-value 0.002; "Exposure to NNRTI (per additional year)" was associated with 1.00 (0.93-1.09) Relative Rate 96% CI, p=0.92). Note: this is where I think James Stein said the confidence intervals overlap). The risks associated with risk factors are noteworthy: current smokers: 2.92 (2.04-4.18), p<0.01; former smokers: 1.63 (1,07-2.48), p=0.02; diabetes: 1.86 (1.31-2.65), p<0.001; hypertension: 1.30 (0.99-1.72), p=0.06; total cholesterol (permmol/liter increase): 1.26 (1.19-1.35), p<0.001; HDL cholesterol .072 (0.52-0.99, p=0.05. I can see why James Stein says these study results are "unclear".
--The primary model (model 1) was adjusted for sex, cohort, HIV transmission group, race or ethnic group )all fixed at enrollment in DAD), age, BMI, family history of cardiovascular disease, smoking status, previous cardiovascular event, and calendar yeat )all-timeupdated to allow a patientfs status to change over time).
--In an additional exploratory model (model 2), we assessed the role of established risk factors for myocardial infarction that may potentially be influenced by ART, including the levels of serum lipids (tptal cholesterol and HDL cholesterol), hypertension, and the presence of diabetes. For each variable, the patientfs most recent measurement at the start of each month was incorporated into the model in a time-updated manner (this analysis excluded patients with missing information on covariates). Thus, the latter analysis allowed us to assess potential mechanisms for any apparent relationship between myocardial infarction and exposure to therapy. The model was also adjusted for the same factors as model 1.
There was no association between either the nadir CD4+ lymphocyte count (relative rate for each increase of 50 cells per cubic millimeter, 0.98; 95% CI, 0.95 to 1.01) or peak HIV-1 RNA level (relative rate for each log10 increase in the number of copies per milliliter, 1.06; 95% CI, 0.95 to 1.18) and the risk of myocardial infarction. Adjustment for these variables did not modify the association between exposure to protease inhibitors and the risk of myocardial infarction.
Discussion
Using a data set that has accrued almost three times more end points in the 3 years of follow-up since the last report,5 we continue to observe an association between exposure to combination antiretroviral therapy and the risk of myocardial infarction. The relationship between exposure to protease inhibitors and myocardial infarction remained significant after multivariable adjustment and was similar to the relationship between exposure to any combination antiretroviral therapy and the risk of myocardial infarction. In contrast, exposure to nonnucleoside reverse-transcriptase inhibitors was not independently associated with an increase in the risk of myocardial infarction. Although experience with nonnucleoside reverse-transcriptase inhibitors remains relatively limited, our results suggest that the previously reported finding of an excess risk in those with increased exposure to combination antiretroviral therapy is likely to be a consequence of exposure to the drugs of the protease-inhibitor class.
Protease inhibitors are known to increase total cholesterol and low-density lipoprotein cholesterol levels to a greater extent than do nonnucleoside reverse-transcriptase inhibitors, whereas the latter class of agents is known to increase HDL cholesterol levels markedly.15,16,17 However, the increased risk of myocardial infarction associated with the use of protease inhibitors seen in our analysis was not fully explained by the lipid changes induced by the drugs in this class. Thus, the full mechanism by which protease inhibitors may lead to increased rates of myocardial infarction remains to be elucidated. Recently published experimental data from murine models suggest possible direct cellular mechanisms by which HIV protease inhibitors may promote atherosclerosis.18,19 Furthermore, there are considerable differences among the various protease inhibitors in their propensity to cause dyslipidemia.16,20 The DAD study currently has insufficient follow-up observations to determine whether the risk of myocardial infarction differs for individual agents.
Some studies have reported that among patients receiving antiretroviral therapy, the degree of dyslipidemia or the risk of coronary heart disease is higher in women21,22 or in younger patients.23 In our analysis, we did not find any evidence that the therapy-attributable risk differs according to age or sex.
In our study, the relative rate of myocardial infarction was 1.16 per year of combination antiretroviral therapy, which corresponds to a doubling of the risk over a 5-year period of exposure. The magnitude of this association is similar to the increment in risk attributable to diabetes mellitus or cigarette smoking and is greater than that associated with a family history of cardiovascular disease. Whether this effect translates into an important additional absolute risk in a person depends on his or her preexisting cardiovascular-disease risk profile. In this regard, our analysis confirmed that the expected associations of established cardiovascular risk factors with myocardial infarction are also seen in the patient population of the DAD study, but our models were not intended to provide the formal basis for calculating individual cardiovascular risk.
We found no association between either the peak HIV-1 RNA level or the nadir CD4+ lymphocyte count and the risk of myocardial infarction, although the possibility that other unmeasured immunologic effects may exert an influence on the development of cardiovascular disease cannot be excluded. The Strategies for Management of Antiretroviral Therapy (SMART) study found that the risk of opportunistic disease or death from any cause was higher in patients whose antiretroviral therapy was interrupted when their CD4+ lymphocyte count reached a certain level than in patients who received continuous treatment.24 Surprisingly, the interruption strategy was also associated with a trend toward an excess risk of cardiovascular disease. The reasons for this finding remain unclear. Theoretically, it could be due to arterial inflammation, a reduction in HDL cholesterol levels, or both caused by an acute-onset increase in viral replication upon interruption of antiretroviral treatment.25,26,27,28 The latter effect may be particularly important after discontinuation of nonnucleoside reverse-transcriptase inhibitors, since these may in themselves cause a rise in HDL cholesterol level.15,16,17
Only a randomized trial, which is not feasible in this setting, could determine whether the observed associations of exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors with the risk of myocardial infarction are causally related. The DAD study, which is observational in design, relies on adjustment of the analyses of association for variables that could potentially act as confounders for these associations. In our analysis, we made adjustments for patient demographics and for the established cardiovascular risk factors. We also adjusted for calendar year to account for secular changes in the management of cardiovascular disease and in the use of antiretroviral agents.
However, there are likely to be other confounding factors that are unknown or are not routinely or easily identified or measured. One possible interpretation of the significant relationship between the risk of myocardial infarction and the length of exposure to combination antiretroviral therapy may be simply that patients with longer periods of exposure to therapy have also been infected with HIV for longer periods. However, our findings that neither the nadir CD4+ count nor the peak HIV-1 RNA level was associated with the risk of myocardial infarction would argue against this interpretation, as would the finding that the risk is different for exposure to nonnucleoside reverse-transcriptase inhibitors and exposure to protease inhibitors.
Another possible concern in observational studies of this type is channeling bias. In our study, channeling bias could have occurred if patients perceived to be at highest risk for cardiovascular disease were either started on or switched to regimens thought to be cardioprotective. Such a bias would tend to reduce the strength of the association of protease-inhibitor exposure with the risk of myocardial infarction. The fact that our analyses are adjusted for most known risk factors for cardiovascular disease reduces the likelihood that channeling has unduly influenced our results. In addition, we assessed the relationship of myocardial infarction with cumulative exposure to an antiretroviral drug rather than with current treatment with an antiretroviral drug, which further reduces the chance of significant bias. Finally, other analyses from the DAD study29 suggest that changes in therapy made because of concern about cardiovascular disease are unlikely to have been frequent enough to bias our results.
In conclusion, this analysis confirmed our previous observation of a significant association between the duration of exposure to combination antiretroviral therapy and the risk of myocardial infarction. In addition, our findings suggest that this effect of antiretroviral therapy varies according to drug class; we found a significant association between exposure to protease inhibitors and the risk of myocardial infarction but no significant association between nonnucleoside reverse-transcriptase inhibitors and such a risk. The effect of protease inhibitors identified in our study may be in part a consequence of the effects of these agents on serum lipid profiles.
Methods
Study Design
DAD is an international collaboration of 11 cohorts of investigators following 23,437 HIV-1-infected subjects at 188 clinics in 21 countries in Europe, the United States, and Australia. Details of the methods have been reported previously.5,12 Patients were followed prospectively during their regular visits to outpatient clinics. All participants were under active follow-up in their cohorts at the time of enrollment in the study (from December 1999 through April 2001). Data on sociodemographic features, clinical findings, treatment (antiretroviral and other medications received both before and after enrollment), and laboratory results were collected at enrollment and at least every 8 months thereafter with the use of standardized forms. All information was transformed into a standardized format and merged into a central data set annually. Institutional review board approval for the study and written informed consent from the study participants were obtained for each cohort individually, according to national and local regulations.
Outcomes
All incident cases of myocardial infarction were reported to the study coordinating office for validation and coding.5 Cases of myocardial infarction were categorized as definite, possible, or unclassifiable (e.g., sudden death in persons with no known terminal condition) and as fatal or nonfatal according to criteria applied in the World Health Organization Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) Study.13,14 Event validation and coding were performed without knowledge of the antiretroviral-treatment history of a patient.
Statistical Analysis
The DAD Steering Committee specified in 1999 that analyses assessing the risk of myocardial infarction according to antiretroviral-drug class would be performed once sufficient events had accumulated to permit such analyses. Details of the analytic approach have been described previously.5 Patients were followed prospectively from enrollment in DAD to the date of the first myocardial infarction, the date of death, 6 months after the patient's last clinic visit, or February 1, 2005, whichever occurred first. Each patient's follow-up period was divided into consecutive 1-month periods, and the patient's cumulative exposure to therapy at the start of each period was calculated (including exposure to treatment before enrollment). This information was used to assign the patient-month (and any events that occurred during that month) to the appropriate exposure category. All analyses were based on the first myocardial infarction that occurred during follow-up. All P values are two-sided, and no adjustment was made for multiple testing.
Poisson regression models (GENMOD procedure, SAS software, version 8) were used to quantify the relationship between exposure to each drug class and the incidence of myocardial infarction. First, we reassessed the association with any combination antiretroviral therapy (i.e., a regimen of more than one antiretroviral drug, including at least one protease inhibitor, at least one nonnucleoside reverse-transcriptase inhibitor, or both). The cumulative exposure to combination antiretroviral therapy was categorized as no exposure, less than 1 year, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 7 years, and more than 7 years and was incorporated as a continuous variable in subsequent analyses.
We then considered the unadjusted relationship between the incidence of myocardial infarction and cumulative exposure to protease inhibitors and nonnucleoside reverse-transcriptase inhibitors. The primary Poisson regression models were then adjusted for demographic factors, calendar year, and conventional risk factors for cardiovascular disease. Specific categories were generated for missing data (e.g., unknown family history) to ensure that all patients and observed events were included. In addition, in sensitivity analyses, we considered the relationships with cumulative exposure to either drug class after exclusion of patients exposed to the other drug class.
In additional analyses, we assessed the role of established risk factors for myocardial infarction that may potentially be influenced by antiretroviral therapy, including diabetes or hypertension and the time-updated levels of serum lipids (total cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides [log2-transformed]). Lipid measurements were included regardless of fasting status. We also assessed the influence of markers of HIV-1 infection (CD4+ lymphocyte count nadir and peak HIV-1 RNA level).
|
|
| |
| |
|
|
|