| |
Switch to Unboosted Reyataz Maintains Viral Suppression, Improves Lipids SWAN Study 48 Weeks Published in CID
|
| |
| |
"Efficacy and Safety of Atazanavir-Based HAART in Patients with Virologic Suppression Switched from a Stable, Boosted or Unboosted Protease Inhibitor Treatment Regimen: The SWAN Study (AI424-097) 48-Week Results'
Clinical Infectious Diseases June 1, 2007
Jose Gatell,1 Dominique Salmon-Ceron,2 Adriano Lazzarin,3 Eric Van Wijngaerden,4 Francisco Antunes,6 Clifford Leen,7 Andrzej Horban,8 Victoria Wirtz,9 Linda Odeshoo,9 Monique Van den Dungen,5 Claudia Gruber,5 and Emilio Ledesma,9,a for the SWAN Study Groupb
1Hospital Clinic-IDIBAPS, University of Barcelona, Barcelona, Spain; 2Hospitalier Cochin, Paris, France; 3San Raffaele Turro, Milan, Italy; 4Universitaire Ziekenhuizen, Leuven, and 5Pharmaceutical Research Institute, Braine l'Alleud, Belgium; 6Instituto Bento da Rocha Cabral, Lisbon, Portugal; 7University of Edinburgh, Scotland; 8Wojewodzki Hospital, Warsaw, Poland; and 9Bristol-Myers Squibb, Pharmaceutical Research Institute, Wallingford, Connecticut
".....This study demonstrated that, in patients with virologic suppression who received a stable PI-based antiretroviral regimen who did not have a history of prior virologic failure of PI therapy, a switch to a simplified PI-based regimen containing atazanavir provided better maintenance of virologic suppression (as demonstrated by significantly lower rates of virologic rebound and treatment failure than those observed with continued, unmodified therapy), comparable safety and tolerability, and improvements in lipid parameters through 48 weeks. Based on these results, the treatment simplification strategy of switching patients to once-daily atazanavir can provide an effective and well-tolerated treatment option...."
Figure 4. Changes from baseline in lipid parameters. HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PI, protease inhibitor; TC, total cholesterol.
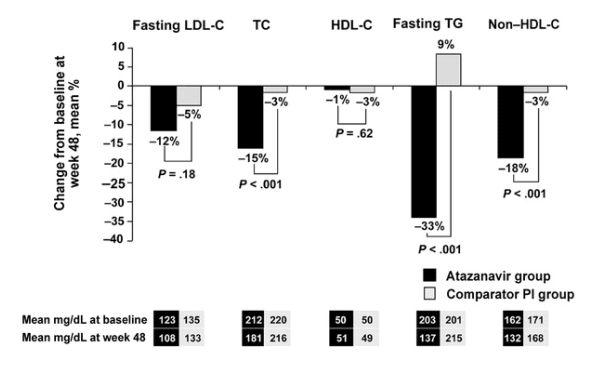
Plasma lipid parameters were comparable at entry between the 2 treatment groups (figure 4). At baseline, 27% of patients in the atazanavir group and 33% of patients in the comparator PI group had elevated serum lipid levels (defined as total cholesterol >240 mg/dL). Patients who switched to atazanavir therapy had reductions in fasting lipid concentrations (total cholesterol, low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and triglycerides) through week 48 (figure 4). Significant differences in mean percentage changes from baseline between treatment regimens at week 48 were reported for total cholesterol, non-high-density lipoprotein cholesterol, and fasting triglyceride levels (P < .001 for all) (figure 4). The proportion of patients who achieved lipid levels below the National Cholesterol Education Program threshold for intervention with pharmacologic lipid-lowering therapy [10] was increased, mainly in the atazanavir group. At week 48, the percentages of patients with low-density lipoprotein cholesterol levels of >100 mg/dL but <130 mg/dL, >130 mg/dL but <160 mg/dL, >160 mg/dL but <190 mg/dL, and >190 mg/dL were 40%, 15%, 5%, and 2%, respectively, for the atazanavir-treatment group, compared with 24%, 24%, 17%, and 11%, respectively, for the comparator PI group. Use of lipid-lowering agents was observed to be higher among patients receiving the comparator PI regimen than among those who were not (14% vs. 8%; P = .05).
ABSTRACT
Background. Atazanavir is a once-daily protease inhibitor (PI) for the treatment of human immunodeficiency virus (HIV) infection that has previously been studied in cohorts of treatment-naive and treatment-experienced patients. Limited data are available on the usefulness of switching from a PI-based regimen to a regimen based on a different PI, such as atazanavir, in HIV-infected patients experiencing virologic suppression but seeking regimen simplification.
Methods. The Switch to Another Protease Inhibitor (SWAN) study was a 48-week, open-label trial involving HIV-positive patients with virologic suppression who were receiving stable PI-based regimens (with or without ritonavir). Patients were randomized 2 : 1 to switch to atazanavir (400 mg per day)-or, if they were receiving tenofovir, to atazanavir-ritonavir (300/100 mg per day)-or to continue to receive their existing PI. The proportion of patients who experienced virologic rebound (defined as an HIV RNA load >50 copies/mL) was compared through study week 48.
Results. Patients either received an atazanavir-containing regimen (278 patients) or continued to receive a comparator PI-containing regimen (141 patients). The proportion of patients who experienced virologic rebound was significantly lower among those who switched to an atazanavir-containing regimen (19 [7%] of 278) than it was among those who continued to receive a comparator PI regimen (22 [16%] of 141; P = .004). Patients who switched to atazanavir therapy experienced significantly fewer total cholesterol, fasting triglyceride, and non-high density lipoprotein cholesterol elevations than did patients in the comparator PI group (P < .001); patients receiving atazanavir had comparable rates of adverse event-related discontinuation and serious adverse events.
Conclusions. In patients with virologic suppression who were receiving other PIs, switching to a once-per-day regimen containing atazanavir provided better maintenance of virologic suppression (as demonstrated by significantly lower rates of virologic rebound and treatment failure than those observed with continued unmodified therapy), a comparable safety profile, and improved lipid parameters, compared with those for patients who continued their prior PI-based regimen through 48 weeks.
HAART has significantly decreased morbidity and mortality among and has extended the life expectancy of individuals with HIV infection [1]. Adherence to HAART regimens is essential for successful virologic and treatment outcomes [2]. The incidence of treatment-limiting adverse events, the frequent dosing schedule, and the effect of a high daily pill burden (with associated food requirements) render many individuals unable to maintain adherence to HAART for extended periods of time [3, 4].
Protease inhibitors (PIs) are commonly used as a critical component of HAART, but the pharmacologic and pharmacokinetic characteristics (including limited oral bioavailability and complex dosing schedules) of many drugs in this class may create challenges for patient compliance [5, 6].
Atazanavir is a potent PI with a pharmacokinetic profile that allows once-daily oral administration with or without ritonavir boosting. Atazanavir, administered at a dosage of 400 mg per day, showed efficacy similar to that of efavirenz and nelfinavir in treatment-naive patients [7, 8]. Atazanavir-ritonavir, administered at 300/100 mg per day, has demonstrated long-term efficacy comparable to that of lopinavir-ritonavir in patients who have experienced multiple virologic failures [9].
The SWAN study explores whether patients who were receiving stable suppressive therapy (their first or second HAART regimen and no prior history of PI failure) that included a boosted or unboosted PI would maintain virologic suppression through 48 weeks after switching to an atazanavir-containing regimen. Switching from a more complex PI-based regimen to a simpler, atazanavir-based regimen may be associated with better gastrointestinal tolerability, improved plasma lipid parameters, and potential clinical benefits for patients.
AUTHOR DISCUSSION
This study demonstrated that, in patients with virologic suppression who received a stable PI-based antiretroviral regimen who did not have a history of prior virologic failure of PI therapy, a switch to a simplified PI-based regimen containing atazanavir provided better maintenance of virologic suppression, comparable safety and tolerability, and improvements in lipid parameters through 48 weeks. Based on these results, the treatment simplification strategy of switching patients to once-daily atazanavir can provide an effective and well-tolerated treatment option.
This study was adequately powered, and the atazanavir treatment regimen was determined to be noninferior to the comparator PI regimen. The proportion of randomized patients who experienced virologic rebound (HIV RNA load, >50 copies/mL) and the proportion of randomized patients who experienced treatment failure through week 48 were both significantly lower in the atazanavir treatment group (P = .004 for both). These results were confirmed with additional analyses involving the subset of patients who received study treatment. In addition, the proportion of patients who experienced virologic rebound and the proportion of patients who experienced treatment failure were significantly lower for patients in the atazanavir group who entered the study receiving an unboosted PI. Patients receiving atazanavir who entered the study receiving a boosted PI demonstrated rates of virologic rebound and treatment failure comparable to those for patients who continued to receive a boosted comparator PI.
The ability of therapy with unboosted atazanavir to maintain virologic suppression after a switch from both ritonavir-boosted or unboosted PI-based regimens may be based on a number of factors, including a limited history of prior antiretroviral therapy and adherence to medication. In this study, patients who had experienced previous virologic failure while receiving a PI-based regimen were excluded. As was previously demonstrated, therapy with unboosted atazanavir may not always be appropriate for patients with a prior history of suboptimal antiretroviral treatment [11].
Overall, the atazanavir and comparator PI regimens exhibited similar safety, similar tolerability, and similar rates of adverse event-related discontinuation. As expected, there was a higher rate of reported scleral icterus and jaundice among patients receiving atazanavir; however, treatment discontinuations related to scleral icterus or jaundice were infrequent. Elevations of liver transaminase levels (alanine aminotransferase and aspartate aminotransferase levels) were infrequent in patients switching to atazanavir therapy and were similar to those for patients who continued unchanged therapy, regardless of coinfection with hepatitis B virus and/or hepatitis C virus. Patients in the comparator PI group reported more gastrointestinal adverse events and used antidiarrheal agents more frequently than patients who switched to atazanavir therapy.
One major difference between treatment groups was that a switch to atazanavir therapy was associated with an improvement in lipid profiles. At week 48, patients in the atazanavir group had statistically significant improvements in the mean percentage change from baseline for total cholesterol, fasting triglyceride, and non-high-density lipoprotein cholesterol levels. More patients who switched to atazanavir therapy than patients who continued to receive their comparator PI achieved lipid levels below the National Cholesterol Education Program-specified threshold for pharmacologic intervention. In addition, lipid-lowering agents were used more frequently in the comparator PI group. Overall, a switch to atazanavir therapy demonstrated comparable efficacy, safety, and tolerability but was associated with an improvement in lipid profiles in a clinical trial that did not require lipid-related abnormalities as study entry criteria. The improved lipid parameters observed in this study are also consistent with findings from earlier trials that evaluated both boosted and unboosted atazanavir therapy in a variety of treatment populations [7, 9, 12-14].
The results of this study should be interpreted in view of several limitations in study design. The first limitation is that the study was an open-label, stable-switch study. Although the intent was to enroll patients to simplify their antiretroviral dosing, it is possible that patients may have been somewhat disenchanted with their current PI regimen. This could explain why more patients randomized to remain in the comparator PI group discontinued the study before receiving study treatment.
Another limitation of the study design is that patients were switched to treatment with an unboosted PI. Since this study was conducted, there have been changes to HIV treatment guidelines to recommend boosted PI regimens over most unboosted PI regimens, and this has been reflected in HIV treatment practice; however, these data remain informative for specific patients who may not be able to take or have access to ritonavir.
Finally, this study enrolled patients who entered the study receiving any PI, and results were not prospectively analyzed according to the specific PIs received. In an effort to better understand the patients who entered the study receiving the most frequently used boosted PI regimen in this study (a lopinavir-ritonavir-based treatment regimen), a post-hoc analysis was conducted. Results that were similar to those of the overall study analysis were observed [15].
Future studies may be warranted to address additional questions about this treatment strategy. Specifically, such studies should evaluate a switch from PI-based regimens to ritonavir-boosted atazanavir regimens among patients who had and patients who had not experienced prior virologic failure of PI therapy. It is also unknown how plasma lipid parameters would change after switching from a boosted PI regimen to a boosted atazanavir regimen, as well as how this would vary depending on the specific antiretroviral drug that the patients switched from.
Switching to a simplified PI-based regimen containing atazanavir provided better maintenance of virologic suppression, as demonstrated by significantly lower rates of virologic rebound and treatment failure than those observed with continued, unmodified therapy. Both the atazanavir-containing regimen and the comparator PI regimen exhibited comparable safety and tolerability. Improved lipid parameters were observed in the atazanavir arm of the study and were confirmed through 48 weeks. However, the clinical significance of these changes in lipid parameters has not been determined.
RESULTS
Baseline Characteristics
Baseline demographic and clinical characteristics, as well as data regarding use of antiretrovirals at screening, were comparable between the 2 treatment groups (tables 1-3). Just over one-half of patients were receiving a boosted PI prior to the switch (most commonly, lopinavir-ritonavir), and nearly one-third of the patients had received a prior diagnosis of AIDS. A total of 37 patients (9%) received tenofovir disoproxil fumarate in the screening regimen (26 [9%] for the atazanavir group and 11 [8%] for the comparator PI group).
Patient Disposition
A total of 230 patients (83%) randomized to receive atazanavir and 105 patients (74%) randomized to receive the comparator PI completed the study through week 48 (figure 1). The median duration of enrollment in the study was 48 weeks for patients in the atazanavir group and 48.7 weeks for patients receiving the comparator PI regimens.
Primary and Secondary Efficacy Analyses
Virologic rebound. The noninferiority of the atazanavir regimen to the comparator PI regimen was demonstrated in the primary analysis, because the percentage of randomized patients with confirmed virologic rebound through week 48 was significantly lower for those who switched to the atazanavir regimen than for those who received the comparator PI (virologic rebound, 7% vs. 16%; P = .004) (figure 2). An additional analysis of virologic rebound within the subpopulation of treated patients also indicated the noninferiority of the atazanavir-containing regimen; the percentage of treated patients who had experienced virologic rebound at week 48 was significantly lower for the atazanavir group, compared with the comparator PI group (7% vs. 17%; P = .002).
Figure 2. Randomized patients who experienced virologic rebound (defined as an HIV load 50 copies/mL; A), virologic rebound in randomized patients who received a boosted protease inhibitor (PI) at screening (B), virologic rebound in randomized patients who received an unboosted PI at screening (C), and the percentage of randomized patients who experienced treatment failure (D).
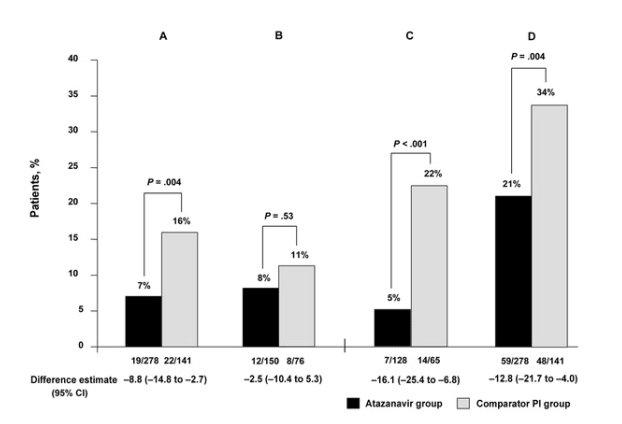
For patients with a history of prior treatment with a boosted PI regimen, the 2 treatment groups had comparable rates of virologic rebound (8% for the atazanavir group vs. 11% for the comparator PI group; P = .53) (figure 2), whereas significantly lower rebound rates were identified within the atazanavir-treatment group, compared with the comparator PI group, within the subpopulation of patients who had received prior unboosted PI therapy (5% vs. 22%; P < .001) (figure 2).
Treatment failure. The proportion of randomized patients who experienced treatment failure through week 48 was significantly lower in the atazanavir group than in the comparator PI group (21% vs. 34%; P = .004) (figure 2 and table 4). An additional analysis restricted to treated patients confirmed the noninferiority of the atazanavir-containing regimen (20% for the atazanavir group vs. 30% for the comparator PI group; P = .03).
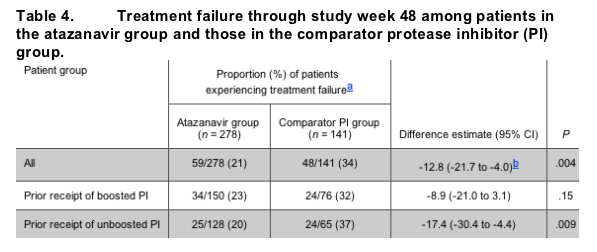
The noninferiority of the atazanavir regimen with respect to treatment failure was also demonstrated for patients having received prior therapy with a boosted PI regimen (23% for the atazanavir group vs. 32% for the comparator PI group; P = .15) and for patients having received prior treatment with an unboosted PI regimen (20% for the atazanavir group vs. 37% for comparator PI group; P = .009) (table 4).
Time to virologic rebound. For randomized patients, the time to virologic rebound was significantly longer for the atazanavir-treated group than for the comparator PI group (P = .007) (figure 3A and table 5). Similar results were identified upon analysis of this end point for the subset of patients who had received prior therapy with an unboosted PI regimen (P = .003). However, no difference was observed between treatment groups with respect to time to virologic rebound for the subset of patients who received a prior boosted PI regimen (P = .47).
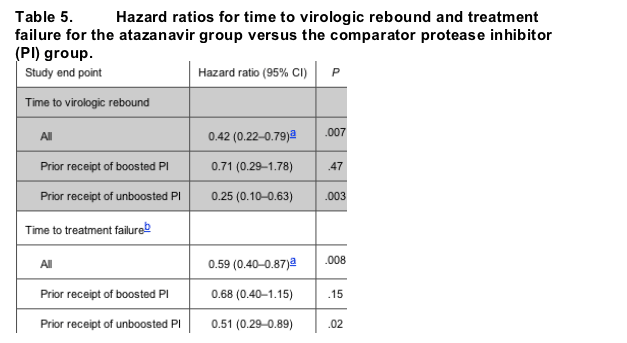
Time to treatment failure. Time to treatment failure was significantly longer for the atazanavir-treated group than for the comparator PI group (P = .008) (figure 3B and table 5) and was significantly longer within the subset of patients who had prior treatment with an unboosted PI regimen (P = .02). No difference was observed for time to treatment failure between treatment groups for patients with a history of prior therapy with a boosted PI regimen (P = .15).
Changes in CD4+ cell count from baseline were comparable between the 2 treatment groups at week 48, with mean increases of 47 cells/mm3 for patients receiving atazanavir and 48 cells/mm3 for patients receiving the comparator PI.
Drug Resistance
Baseline drug-resistance data were not obtained, because all patients had virologic suppression at study entry. Virologic rebound to plasma concentrations of HIV RNA load >1000 copies/mL occurred in 8 (3%) of the randomized patients in the atazanavir group and 7 (5%) of the randomized patients in the comparator PI group; post-treatment failure drug-resistance testing was performed for 4 of those patients in the atazanavir group and 3 of those patients in the comparator PI group. Small numbers of patients and lack of baseline drug-resistance data do not allow for meaningful interpretation of these results; however, among patients for whom drug-resistance testing was performed, there were no apparent differences in the rate of drug-resistance mutations observed.
Safety
There were no deaths within the atazanavir-treatment group and 5 deaths in the comparator PI group (table 6). Of the 5 deaths in the comparator PI group, 1 occurred after randomization but prior to study treatment, and 1 occurred after week 48. Causes of death included septic shock, malignancy, depression, and overdose of a narcotic drug. No information on causality could be obtained for the fifth death, and no deaths were attributed to study treatment.
The incidence of serious adverse events was 10% in the atazanavir group and 6% in the comparator PI group (table 6). Serious adverse events that were reported for 5 patients who were treated with atazanavir were considered to be related to receipt of the study drug.
The adverse event-related discontinuation rate was comparable for both regimens (6%). Grade 2-4 jaundice or scleral icterus was reported for 3% of patients in the atazanavir group (table 6) and was considered to be related to study therapy. Grade 3 or 4 jaundice and/or scleral icterus was reported in 5 (2%) of atazanavir-treated patients and was associated with treatment discontinuation in 3 patients.
Gastrointestinal adverse events of any grade (e.g., diarrhea, nausea, vomiting, and abdominal pain) occurred more frequently among patients within the comparator PI group (13%) than among patients treated with atazanavir (8%). There was also a significantly lower rate of antidiarrheal drug use for patients in the atazanavir group, compared with the comparator PI group (2% vs. 7%; P = .04).
Laboratory Evaluations
Grade 3 or 4 alanine aminotransferase and aspartate aminotransferase elevations occurred infrequently in both groups (table 7). A higher incidence of elevated alanine aminotransferase levels was observed among patients with hepatitis B virus or hepatitis C virus coinfection and was comparable in frequency between groups (14% in the atazanavir group vs. 16% in the comparator PI group). Total bilirubin levels were higher in patients who switched to atazanavir therapy for both the overall patient population and the hepatitis-coinfected population.
METHODS
Study design. This was a 48-week, open-label trial enrolling HIV-positive patients with virologic suppression who were receiving stable PI-based regimens with or without ritonavir (figure 1). The study was approved by the internal review boards at each site, and all patients gave written informed consent. Patients were randomized 2 : 1 either to receive an atazanavir-containing regimen or to continue their current PI-containing regimen. Patients in the switch group were to receive unboosted atazanavir (400 mg once per day) instead of their current PI. When tenofovir disoproxil fumarate was used as a part of the nucleoside backbone at study entry, patients were switched to atazanavir-ritonavir (300/100 mg per day), but the other components of the HAART regimen remained unchanged. Randomization of patients who were receiving a ritonavir-boosted PI at study entry was limited to 50% of the sample size.
Inclusion criteria. Prospective patients were required to be receiving a stable PI-containing regimen (their first or second HAART regimen) with or without ritonavir. Patients with a history of virologic failure while receiving PI-based HAART were excluded from the study. Patients who had experienced previous virologic failure who received a nonnucleoside reverse-transcriptase inhibitor regimen were eligible for inclusion in the study. Patients were required to have a suppressed plasma viral load (defined as an HIV RNA load of <50 copies/mL) for >3 months prior to screening and a CD4+ cell count >50 cells/mm3. The current PI component(s) of the patients' therapy had to be dosed at least twice daily and/or include >3 pills per day.
Efficacy end points. A total of 372 randomized patients provided 90% power to demonstrate the noninferiority of the atazanavir regimen to the comparator PI regimen. This calculation assumed a 2-sided 95% CI, an upper confidence limit of 12%, and a virologic rebound rate of 13% for the comparator PI regimen.
The primary efficacy end point was the proportion of randomized patients who experienced virologic rebound (i.e., the proportion of randomized patients with confirmed on-study HIV RNA loads >50 copies/mL or last on-study HIV RNA load 50 copies/mL followed by study discontinuation) at or prior to week 48. Secondary efficacy end points included time to virologic rebound and mean change from baseline CD4+ cell count through week 48. Post-hoc analyses included the proportion of patients who experienced treatment failure (virologic rebound, never initiating study therapy, or discontinuation of study therapy) and time to treatment failure through week 48.
Safety end points. Safety end points included the frequency and severity of adverse events, serious adverse events, abnormal laboratory results, and adverse event-related discontinuations. Additional measures were mean percentage changes from baseline in total cholesterol, fasting low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and fasting triglyceride levels at week 48.
Data analysis. The efficacy data set included data from all randomized patients. Treatment comparisons were made using the difference in proportions (values for the atazanavir regimen minus values for the comparator PI regimen) stratified by prior PI regimen (ritonavir-boosted regimen vs. unboosted regimen) with a 95% CI and a P value based on normal approximations. An atazanavir-containing regimen was determined to be noninferior to the comparator PI regimen if the upper confidence limit of the difference in proportions of virologic rebound was <12%. In addition to the planned analyses, additional analyses were performed for the subset of treated patients.
The safety data set included all patients receiving >1 dose of the study therapy. Adverse events and laboratory measurements were included through 30 days after the last dose of study therapy (atazanavir or comparator PI). The investigators determined the intensity of adverse events using a modified World Health Organization grading system and the relationship of adverse events to study therapy. Serious adverse events and deaths were included without regard to treatment status at the time of onset for enrolled patients.
Absolute and percentage changes in lipid levels were summarized by treatment regimen through week 48 for treated patients. Percentage changes from baseline for lipid parameters were computed on the log scale and back transformed. Treatment regimens were compared using the week 48 difference (values for the atazanavir regimen minus values for the comparator PI regimen) in mean percentage changes for each parameter using a 95% CI and a P value based on Student's t tests. Lipid data were excluded from analyses after the initiation of therapy with serum lipid-lowering agents.
|
|
| |
| |
|
|
|