| |
Kaletra Efficacy with Low Adherence Levels
|
| |
| |
"HIV-Infected Patients Receiving Lopinavir/Ritonavir-Based Antiretroviral Therapy Achieve High Rates of Virologic Suppression Despite Adherence Rates Less Than 95%"
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 45(1)1 May 2007pp 4-8
Shuter, Jonathan MD; Sarlo, Julie A PA*; Kanmaz, Tina J PharmD; Rode, Richard A PhD; Zingman, Barry S MD
".....In the present study, 80% of lopinavir/ritonavir recipients achieved VLs <400 copies/mL and 59% achieved VLs <75 copies/mL, despite a mean adherence rate of 73% and substantial antiretroviral experience. Remarkably, in the lowest adherence quartile, with adherence rates ranging from 23.5% to 53.3%, 69% of subjects achieved VLs <400 copies/mL and 56% achieved VLs <75 copies/mL. These rates were virtually identical to those of the overall study cohort..... Lopinavir/ritonavir's forgiveness of nonadherence is likely attributable to 2 separate factors. First is its pharmacokinetic profile. A single 400-mg dose of lopinavir boosted with 50 mg of ritonavir produced lopinavir levels that exceeded the 50% effective concentration (EC50) for HIV for >24 hours in normal volunteers.17 It is thus likely that patients without resistant virus who miss 1 of 2 daily doses of lopinavir/ritonavir could maintain therapeutic levels of the drug between doses. In contrast, the shorter half-lives of unboosted PIs18 increase the likelihood of plasma drug concentrations falling to subtherapeutic levels after each missed dose. Patients missing or delaying a dose of lopinavir/ritonavir are hence more likely to retain therapeutic levels of drug than those missing a dose of an unboosted PI. The second factor that may enhance the forgiveness of lopinavir/ritonavir is the low frequency at which the virus develops resistance to this agent.9,19,20 This characteristic may allow for the sustained efficacy of lopinavir/ritonavir even after substantial cumulative exposure of the virus to subtherapeutic concentrations of the drug...."
Abstract
Background: The observation that extremely high levels of medication adherence are required to achieve complete virologic suppression is based largely on studies of treatment-experienced patients receiving HIV protease inhibitor (PI)-based therapy without ritonavir boosting. This study aims to define the level of adherence needed to achieve virologic suppression in patients receiving boosted PI-based highly active antiretroviral therapy (HAART) with lopinavir/ritonavir.
Methods: HIV-infected adults receiving a regimen containing lopinavir/ritonavir were recruited into a prospective, observational study of the relation between adherence to lopinavir/ritonavir and virologic outcomes. Adherence was measured using the Medication Event Monitoring System (MEMS; Aardex, Union City, CA). HIV-1 viral load (VL) was measured at week 24.
Results: The final study population contained 64 subjects. Eighty percent had AIDS, 97% received lopinavir/ritonavir before enrollment, and most had more than 7 years of HAART experience. Mean adherence overall was 73%. Eighty percent and 59% achieved a VL <400 copies/mL and a VL <75 copies/mL, respectively. Mean adherence was 75% in those achieving a VL <75 copies/mL. High rates of virologic suppression were observed in all adherence quartiles, including the lowest quartile (range of adherence: 23.5%-53.3%).
Conclusions: Moderate levels of adherence can lead to virologic suppression in most patients taking lopinavir/ritonavir-based HAART.
Maintaining excellent adherence to antiretroviral therapy is a cornerstone of HIV infection management. Although it is advisable for patients to adhere faithfully to all prescribed medications, it is possible that some HAART regimens are more forgiving of imperfect adherence than others.
The Medication Event Monitoring System (MEMS; Aardex, Union City, CA) provides a means of quantitating pill ingestion over time by electronically recording bottle openings. MEMS monitors (caps) are more sensitive than other commonly used methods for detecting nonadherence and have been shown to be more accurate at predicting virologic outcomes in HIV-infected patients receiving highly active antiretroviral therapy (HAART).1,2
Three early studies using MEMS caps to assess adherence to HAART demonstrated that adherence rates of 90% to 98% were necessary to achieve complete virologic suppression in a majority of patients.3-5 Most patients in these studies received regimens consisting of 2 nucleoside reverse transcriptase inhibitors and 1 HIV protease inhibitor (PI) without ritonavir boosting (unboosted PI). Their findings led to the acceptance of the 95% rule for HAART adherence (ie, that patients must take ≥95% of prescribed doses to achieve complete virologic suppression) by the scientific and lay HIV care community.6,7
The improved pharmacokinetic profiles of newer antiretroviral agents, largely attributable to ritonavir boosting, raise the prospect of greater forgiveness of missed doses. A recent report used pharmacy refill data to show that boosted PI-based HAART is more forgiving of nonadherence than other treatment strategies.8 The improved pharmacokinetic properties of lopinavir/ritonavir over unboosted PIs and the low likelihood of viral resistance, even in the setting of suboptimal adherence,9 suggest that lopinavir/ritonavir-based HAART may be more forgiving of nonadherence than earlier unboosted PI-based regimens. We therefore conducted a study to evaluate the association between adherence to lopinavir/ritonavir and virologic outcomes in a group of urban HIV-infected adults.
METHODS
Montefiore Medical Center's Moses Division is a 708-bed tertiary care teaching hospital located in the Bronx, New York. The hospital's Center for Positive Living/Infectious Diseases Clinic provides comprehensive outpatient care to more than 2500 HIV-infected adults, primarily from the surrounding areas of the Bronx. Most patients who visit the clinic belong to ethnic minority groups and have incomes below the federal poverty line. In 2005, 60% of clinic patients had Centers for Disease Control and Prevention (CDC)-defined AIDS, and 70% to 80% were prescribed HAART.
Patients were enrolled in the study between May 2004 and November 2005. Eligibility criteria included the following: (1) HIV infection confirmed by enzyme immunoassay or plasma HIV-1 RNA assay; (2) initial or maintenance treatment with lopinavir/ritonavir therapy at a dose of 3 or 4 133-mg/33-mg soft-gel capsules twice daily (once lopinavir/ritonavir 200-mg/50-mg tablets became available, subjects receiving 2 tablets twice daily were also permitted to participate in the study); (3) likelihood, in the judgment of the patient and his or her provider, that lopinavir/ritonavir therapy would continue for the ensuing 24 weeks; (4) willingness to cap the lopinavir/ritonavir bottle with a MEMS monitor and to take all doses, 1 dose at a time, from this bottle; and (5) willingness to provide informed consent and to complete the 5 scheduled study visits.
Study subjects were instructed in the proper use of the MEMS caps (eg, open the cap once and only once at each dose time, do not take doses out in advance for later use or to load a pillbox, time the switch-over of the cap to dose time when a new bottle of lopinavir/ritonavir is obtained from the pharmacy). Subjects were to complete 4 study visits after the initial visit at 2 weeks, 8 weeks, 16 weeks, and 24 weeks. At each study visit, data collected by means of MEMS caps were downloaded into the study computer and then uploaded (stripped of all patient-identifying data) to a server at Aardex. At each visit, the study staff administered a standardized questionnaire pertaining to MEMS cap use and time periods during which MEMS cap use was suspended while the subject continued taking lopinavir/ritonavir (eg, hospitalization, incarceration). The results of these questionnaires were anonymized and transmitted to Aardex so that periods of improper or lapsed use of MEMS caps could be excluded from analysis. All subjects had an HIV-1 RNA assay (viral load [VL]) measurement at enrollment and another VL measurement on or about week 24 (a 2-week window period on either side of the week 24 time point was permitted) of the study. Between May 2004 and August 2005, VLs were measured by reverse transcriptase polymerase chain reaction (RT-PCR) using the Roche Amplicor 1.5 assay (Roche Diagnostics, Pleasanton, CA), which has a lower limit of detection of 50 copies/mL. In August 2005, the Montefiore Medical Center Clinical Laboratory switched to the Bayer Versant branched-DNA (bDNA) version 3.0 (Bayer Healthcare LLC, Berkeley, CA), which has a lower limit of detection of 75 copies/mL. From August 2005 until the end of the study, VLs were measured with the latter assay. For the sake of uniformity, a VL <75 copies/mL was considered complete virologic suppression for all subjects. At the week 24 study visit, subjects completed a previously validated Adult AIDS Clinical Trial Group (AACTG) adherence instrument, which quantitates adherence by 4-day recall.10 Subjects who completed the baseline and week 24 visits and who had VL measurements at both time points were entered into the final analysis. Subjects who discontinued lopinavir/ritonavir, refused to use the MEMS cap properly throughout the 24-week study period, broke or lost more than 1 MEMS cap, or failed to attend the week 24 visit were excluded from the final analysis.
Basic demographic and clinical information was collected from each study subject during a brief interview and from medical record review at study enrollment. MEMS data were compiled and cleaned by Aardex and transmitted directly to the principal investigator (JS), independent of the data transfer to the study sponsor. Medication adherence was defined as: (number of bottle openings/number of prescribed doses of lopinavir/ritonavir) X 100, excluding all time periods that subjects reported taking lopinavir/ritonavir but not using the MEMS caps. Four-day self-reported adherence was calculated according to the formula: (number of complete doses of antiretroviral regimen reported taken/number of doses prescribed) X 100. VL measurements were accessed through the clinical information system of the hospital. All the study measures were entered into a secure computerized database and exported to SPSS version 14.0 (SPSS, Chicago, IL) for analysis.
Comparisons of proportions (percentages) of subjects achieving virologic suppression in different adherence strata were accomplished using the Fisher exact test. Continuous variables were compared between groups using the Student t test for normally distributed data and the Mann-Whitney U test for data that were not normally distributed. All aspects of the study were reviewed and approved by the Montefiore Medical Center Institutional Review Board.
The study sponsor had no role in subject recruitment or data collection. Data analyses were completed at Montefiore Medical Center, with statistical results independently verified by an employee of the sponsor (RAR, a biostatistician). Members of the funding organization were permitted to make suggestions during manuscript preparation, but final decisions regarding manuscript content and submission were made at the sole discretion of the principal investigator (JS).
RESULTS
A total of 84 patients were enrolled between May 2004 and November 2005. Ten patients discontinued lopinavir/ritonavir during the course of the study for the following reasons: worsening glycemic control (n = 3), gastrointestinal intolerance (n = 2), relapsed substance abuse (n = 3), and unknown reasons (n = 2). Ten subjects who remained on lopinavir/ritonavir failed to complete the study because of inability or unwillingness to use the MEMS caps properly and to complete the study visits. One of these subjects completed 24 weeks of MEMS monitoring but failed to complete the 24-week VL measurement. A total of 64 subjects were available for the final analysis.
Baseline demographic and clinical data on the final study sample are presented in Table 1. We compared the demographic and clinical profiles of the subjects who completed the study with those of the subjects who did not. There were no significant differences between these groups with regard to age, gender, ethnic distribution, HIV risk behavior, years since HIV diagnosis, proportion with CDC-defined AIDS, cumulative duration of prior antiretroviral therapy, lowest documented CD4+ lymphocyte count, highest documented VL, or VL at study enrollment. There was a trend toward a higher CD4+ lymphocyte count at study enrollment in patients who completed the study compared with those who did not (455 vs. 324 cells/μL; P = 0.07).
Sixty-two (96.9%) of the 64 subjects included in the final analysis were receiving lopinavir/ritonavir before study enrollment. The mean duration of MEMS cap monitoring was 167 days, or 23.9 weeks. The mean adherence to lopinavir/ritonavir was 72.8% ± 22.2% (range: 23.5%-100%). Adherence rates in the lowest adherence quartile ranged from 23.5% to 53.3%, in the second quartile from >53.3% to 77.5%, in the third quartile from >77.5% to 92.9%, and in the highest quartile from >92.9% to 100%. Thirteen patients (20.3%) had adherence rates >95%, including 3 patients (4.7%) with adherence rates of 100%. Self-reported adherence data were available for 59 of the 64 subjects. The mean self-reported 4-day adherence was 90.5% ± 19.7%, and 40 subjects (67.8%) reported 100% adherence. Only 6 subjects (10.2%) reported 4-day adherence rates less than 75%. There was a significant correlation between self-reported adherence and adherence as measured by MEMS caps (Spearman ρ = 0.41; P = 0.001).
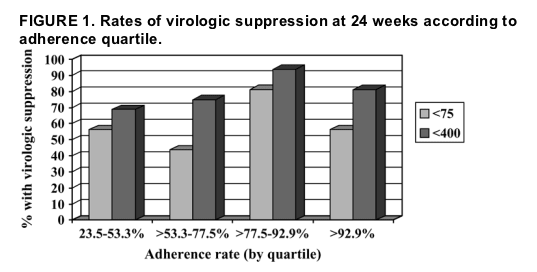
Percentages of patients in each adherence quartile who achieved virologic suppression using cutoffs of <400 copies/mL and <75 copies/mL are shown in Figure 1. There was no statistically significant difference in proportions of subjects with virologic suppression between adherence quartiles for the cutoff of <400 copies/mL (P = 0.42) or for the cutoff of <75 copies/mL (P = 0.18). There seemed to be a trend toward better virologic outcomes among the 32 subjects in the top 50th percentile of adherence (>77.5% adherent) as compared with the 32 subjects in the bottom 50th percentile of adherence (≦77.5% adherent), defined, respectively, by suppression to less than 400 copies/mL (87.5% vs. 71.9%; P = 0.21) and suppression to less than 75 copies/mL (68.8% vs. 50%; P = 0.20). These differences did not achieve statistical significance, however. On bivariate analysis, there was no statistically significant association between VL at 24 weeks and adherence as measured by MEMS caps (Spearman ρ = -0.10; P = 0.46) or by self-report (Spearman ρ = -0.17; P = 0.21).
Fifty-one subjects achieved a VL <400 copies/mL at week 24, and 13 subjects failed to achieve this endpoint. The mean adherence rate among those with a VL <400 copies/mL was 75.1% as compared with 63.6% in those with a VL ≥400 copies/mL (P = 0.09). Mean self-reported adherence among those with a VL <400 copies/mL was 91.9% as compared with 84.1% in those with a VL ≥400 copies/mL (P = 0.24). Thirty-eight subjects achieved a VL <75 copies/mL at week 24, and 26 subjects failed to achieve this endpoint. The mean adherence rate among those with a VL <75 copies/mL was 75.4% as compared with 69.0% in those with a VL ≥75 copies/mL (P = 0.26). Mean self-reported adherence among those with a VL <75 copies/mL was 92.5% as compared with 87.5% in those with a VL ≥75 copies/mL (P = 0.34).
We were not able to correlate adherence with virologic outcomes in the 20 subjects who did not complete the study. Follow-up virologic data were available for all 20 subjects in their medical records, however. Fifteen (75%) of them achieved a VL <75 copies/mL between study enrollment and manuscript preparation.
DISCUSSION
The ideal model of antiretroviral therapy for HIV infection has every patient taking every prescribed dose of medication for the long term. A target adherence rate of 95% or greater for HAART recipients is firmly established in the medical literature.11,12 Patients and their providers dedicate much time and energy trying to realize this ambitious goal. In practical terms, maintaining 95% adherence means that patients receiving once-daily therapy must not miss more than 1 dose per month. Although this is a worthy goal for each individual patient, experience teaches that it is unrealistic for most. In our study cohort, a group of typical urban HIV-infected patients who agreed to undergo 24 weeks of adherence monitoring, only 20% were able to meet or exceed the 95% adherence target.
Participants in this study underwent electronic monitoring of adherence to lopinavir/ritonavir with MEMS caps for a period of 24 weeks. MEMS cap monitoring is a more sensitive test for nonadherence than self-report and is more closely correlated with virologic outcomes than alternative adherence measures.1,2 Although there is no gold standard method of determining adherence, many consider electronic monitoring with MEMS caps to be the most rigorous single strategy. Because data derived from MEMS caps are not comparable to those derived from other common methods of adherence estimation (eg, self-report, pill count),1,2 the discussion refers exclusively to other trials using MEMS cap technology.
Four separate studies of different patient populations receiving primarily unboosted PI-based HAART demonstrated high rates of virologic suppression when adherence exceeded the 90% to 98% range and significant declines in rates of virologic suppression at adherence rates less than 90% to 98%.3-5,13 These studies, similar to our study, enrolled mostly or exclusively patients who were already receiving HAART. The findings of the first 3 of the studies led to the establishment of the 95% rule of antiretroviral adherence.
Three additional studies of various HAART regimens (not including ritonavir-boosted PI regimens) confirmed the direct correlation of virologic suppression with adherence but noted moderate levels of virologic suppression even in those who were not in the top adherence strata.2,14,15 Bangsberg13 recently reported high rates of virologic suppression in recipients of nonnucleoside reverse transcriptase inhibitor (NNRTI)-based HAART at all adherence levels exceeding 53% and concluded that NNRTI-based HAART is more forgiving of nonadherence than unboosted PI-based HAART. Some authors have speculated that ritonavir-boosted PI therapy may be more forgiving of nonadherence than unboosted PI therapy,13,16 but ours is the first study that used electronic adherence monitoring to test the hypothesis. In the present study, 80% of lopinavir/ritonavir recipients achieved VLs <400 copies/mL and 59% achieved VLs <75 copies/mL, despite a mean adherence rate of 73% and substantial antiretroviral experience. Remarkably, in the lowest adherence quartile, with adherence rates ranging from 23.5% to 53.3%, 69% of subjects achieved VLs <400 copies/mL and 56% achieved VLs <75 copies/mL. These rates were virtually identical to those of the overall study cohort.
Lopinavir/ritonavir's forgiveness of nonadherence is likely attributable to 2 separate factors. First is its pharmacokinetic profile. A single 400-mg dose of lopinavir boosted with 50 mg of ritonavir produced lopinavir levels that exceeded the 50% effective concentration (EC50) for HIV for >24 hours in normal volunteers.17 It is thus likely that patients without resistant virus who miss 1 of 2 daily doses of lopinavir/ritonavir could maintain therapeutic levels of the drug between doses. In contrast, the shorter half-lives of unboosted PIs18 increase the likelihood of plasma drug concentrations falling to subtherapeutic levels after each missed dose. Patients missing or delaying a dose of lopinavir/ritonavir are hence more likely to retain therapeutic levels of drug than those missing a dose of an unboosted PI. The second factor that may enhance the forgiveness of lopinavir/ritonavir is the low frequency at which the virus develops resistance to this agent.9,19,20 This characteristic may allow for the sustained efficacy of lopinavir/ritonavir even after substantial cumulative exposure of the virus to subtherapeutic concentrations of the drug.
The present study had several limitations. We did not find the tight association between adherence and virologic suppression described in earlier studies, nor did we find a specific adherence threshold below which extremely high rates of virologic failure occurred. The small sample size may have limited the ability of the study to detect these relations. The attrition rate was almost 24%. This rate of noncompletion is comparable to those of other MEMS cap studies of similar size.1-3 The observation that 75% of noncompleters achieved complete virologic suppression during clinic follow-up argues against the claim that poor adherers with poor virologic outcomes were selectively lost to follow-up. Most subjects entered the study already on lopinavir/ritonavir, raising concerns that lopinavir/ritonavir successes, even in those who had suboptimal adherence, were overrepresented. Although we cannot exclude this possibility, the fact that most study subjects achieved complete virologic suppression after 24 weeks of follow-up despite adherence rates far less than the 95% target remains notable. The study is not informative about the long-term consequences of suboptimal adherence. We presented summary data for adherence rates averaged over 24 weeks of follow-up. This approach does not consider the possibility that poorly adherent patients adhered better in anticipation of their final study visit. Time-dependent analyses of adherence (data not presented) demonstrated that, consistent with other studies,14,21 adherence decreased over the study period, however, making a deceptive increase in virologic suppression at week 24 on this basis less plausible. Additionally, we did not systematically collect information on virologic resistance. It is theoretically possible that resistance to lopinavir/ritonavir was more common in the higher adherence strata, thus tending to level the virologic suppression rates across all strata. This would not explain the high levels of virologic suppression observed in the lower adherence strata, however. Finally, all subjects in the study were receiving lopinavir/ritonavir-based HAART. Findings in our patient sample may or may not be generalizable to patients receiving other modern HAART regimens.
In this study, treatment-experienced patients receiving lopinavir/ritonavir-based HAART achieved high rates of virologic suppression across a wide range of adherence strata. There was no evidence of a significant increase in the rate of virologic failure when adherence rates, as measured by MEMS caps, dropped to less than the 90% to 95% level. These findings challenge the belief that near-perfect adherence is necessary to achieve virologic suppression in persons living with HIV in the current HAART era. Certainly, providers should still counsel their patients to strive for maximal adherence to all prescribed medications. Doubts about the ability of patients to adhere perfectly to their regimens should not dissuade providers from prescribing HAART, however, especially regimens that have demonstrated greater forgiveness of missed doses.
|
|
| |
| |
|
|
|