| |
Protease inhibitor-based antiretroviral therapy and glucose tolerance in pregnancy: AIDS Clinical Trials Group A5084
|
| |
| |
American Journal of Obstetrics and Gynecology
Volume 196, Issue 4, April 2007, Pages 331.e1-331.e7
Presented at the 2006 Conference on Retroviruses and Opportunistic Infections, February 5-8, 2006, Denver, CO.
"In summary, in this prospective observational study, it does not appear that use of protease inhibitors in pregnancy is associated with abnormal glucose tolerance or with subclinical disruption in insulin and glucose homeostasis, despite the well-documented association between protease inhibitors and insulin resistance in nonpregnant adults. One might speculate that placentally mediated insulin resistance occurring in the context of normal pregnancy overwhelms any potential effect of protease inhibitors on insulin resistance in pregnancy. These findings, as well as the lack of adverse pregnancy or neonatal outcomes associated with protease inhibitor use in this report, are reassuring with regard to the widespread use of protease inhibitors during pregnancy for prevention of mother-to-child HIV transmission as well as for maternal health. The possibly increased rate of gestational diabetes observed in this study population is of concern, although it may be related to population characteristics such as high body mass index and race/ethnicity. If corroborated in other cohorts of HIV-infected pregnant women, further study of the relationships among HIV, antiretroviral therapy, body mass index, and gestational diabetes would be warranted."
Jane Hitti MD1, Janet Andersen ScD2, Grace McComsey MD3, Tun Liu MS2, Ann Melvin MD1, Laura Smith BS4, Alice Stek MD5, Judith Aberg MD6, Andrew Hull MD7, Beverly Alston-Smith MD8, D. Heather Watts MD8, Elizabeth Livingston MD9 and AIDS
Clinical Trials Group 5084 Study Team
1University of Washington, Seattle, WA
2Harvard School of Public Health, Boston, MA
3Case Western Reserve University, Cleveland, OH
4Frontier Science Technology and Research Foundation, Amherst, NY
5University of Southern California, Los Angeles, CA
6New York University, New York, NY
7University of California, San Diego, San Diego, CA
8National Institutes of Health, Bethesda, MD
9Duke University, Durham, NC.
ABSTRACT
Objective
The objective of the study was to determine whether protease inhibitors increase glucose intolerance and insulin resistance in pregnancy.
Study Design
In this multicenter, prospective, observational study, 149 human immunodeficiency virus-1-infected pregnant women had fasting insulin, glucose, and C-peptide measured followed by a 1 hour, 50 g glucose test. Glucose intolerance was defined as a 1 hour glucose greater than 130 mg/dL. Glucose intolerance, homeostasis model assessment of insulin resistance and pancreatic _-cell function, and pregnancy outcomes were compared between those taking protease inhibitors and those not.
Results
Fifty-seven of 149 subjects (38%) had glucose intolerance. Body mass index, Hispanic ethnicity, and maternal age, but not protease inhibitors, were associated with glucose intolerance. There were no differences in insulin resistance, _-cell function, or pregnancy outcome associated with protease inhibitor use.
Conclusions
Protease inhibitors do not increase risk of glucose intolerance or insulin resistance among pregnant women.
Insulin resistance leading to impaired glucose tolerance is a well-recognized complication of protease inhibitor-based antiretroviral therapy and likely results from inhibition of glucose uptake by adipocytes and perhaps also impaired insulin secretion by pancreatic _-cells.1, 2, 3, 4 and 5 Protease inhibitors are currently recommended as the mainstay of antiretroviral therapy for human immunodeficiency virus-1 (HIV)-infected pregnant women in the United States.6 However, to date the impact of protease inhibitor antiretroviral therapy on glucose tolerance in pregnancy has only been examined in retrospective studies7, 8 and 9 or in secondary analyses of prospective cohorts.10 and 11
Gestational diabetes complicates 2-5% of all pregnancies12 and occurs as the result of insulin resistance mediated by placental hormones such as human placental lactogen and human placental growth hormone.13 To test the hypothesis that protease inhibitor-containing antiretroviral therapy is associated with an increased rate of abnormal glucose tolerance in pregnancy, we conducted a prospective observational study comparing the combined incidence of abnormal glucose tolerance and gestational diabetes among HIV-infected pregnant women on protease inhibitor-based antiretroviral therapy to that among HIV-infected pregnant women not on protease inhibitors.
We used homeostasis model assessment (HOMA), a model of the glucose/insulin feedback system, to derive estimates of _-cell function (HOMA-_) and insulin resistance (HOMA-IR)14 from paired measurements of fasting glucose and insulin. _-Cell function and insulin resistance measured by HOMA have been shown to correlate with measures obtained by hyperglycemic and euglycemic clamp studies. We examined HOMA-_, HOMA-IR, quantitative insulin sensitivity check index (QUICKI),15 and C-peptide to understand whether abnormal glucose tolerance resulted from insulin resistance and/or decreased insulin production and glycosylated hemoglobin as a measure of long-term glucose control. Finally, we compared gestational age at delivery, mode of delivery, and infant birth weight between groups to explore the effect of protease inhibitor-based antiretroviral therapy on these outcomes.
Materials and Methods
The AIDS Clinical Trials Group Protocol A5084 was a prospective, multicenter, observational cohort study designed to evaluate the safety and tolerance of antiretroviral medications in HIV-1-infected pregnant women, conducted at 28 centers in the United States between 2001 and 2005. The study was approved by the institutional review boards at all centers, and all study subjects provided written informed consent for participation. Written informed consent was also obtained from the father of the fetus, if available.
HIV-infected pregnant women at 20-34 weeksf gestation who had been on stable antiretroviral therapy or no antiretroviral therapy for 8 weeks or more were eligible to participate. Women with diabetes prior to pregnancy were excluded; however, women with a history of gestational diabetes in a prior pregnancy were eligible. Women with multiple gestations, major fetal congenital anomalies, other obstetrical complications, serious bacterial infections, other serious or unstable medical conditions, active drug or alcohol abuse, or recent use of any medication that could interfere with glucose metabolism were not eligible to participate. Enrollment was targeted by type of antiretroviral regimen (n = 80 with protease inhibitors vs n = 80 without protease inhibitors including women receiving no antiretroviral therapy at study entry) and also by gestational age at enrollment (at least 20 subjects in each antiretroviral treatment group at gestation of 26 weeks or less).
Each study visit included documentation of interval medical and obstetric history, concomitant medications, and adherence to antiretroviral medications and blood draws for routine safety and HIV-related laboratory studies. At study entry, subjects had a fasting blood draw for glucose, insulin, and C-peptide, followed by a 50 g oral glucose challenge and a second blood draw 1 hour later for glucose, insulin, and glycosylated hemoglobin.
Abnormal glucose tolerance was defined as a 1 hour glucose value greater than 130 mg/dL. If this occurred, the subject was asked to return for a 100 g, 3 hour glucose tolerance test as described by the American Diabetes Association.16 Gestational diabetes was diagnosed as 2 or greater abnormal glucose values according to the Carpenter and Coustan criteria.17 Women who met criteria for gestational diabetes were treated clinically as indicated and did not have further glucose tests during pregnancy. Those subjects without gestational diabetes had study evaluations repeated every 8 weeks until delivery.
A newborn physical examination was documented within 48 hours of birth, including weight, length, and head circumference. Maternal and infant medical records were abstracted after delivery. Infant follow-up for HIV diagnosis was conducted according to U.S. Public Health Service recommendations.18
Women were asked to return for follow-up 12 weeks after delivery. Postpartum evaluations included a fasting blood draw for glucose, insulin, and C-peptide, followed by a 75 g glucose load and 2 hour blood draw for glucose and glycosylated hemoglobin.
Glucose, glycosylated hemoglobin, complete blood counts, T-cell subsets, and HIV-1 ribonucleic acid quantification were performed real-time at each participating site in certified laboratories. Specimens for insulin were drawn in 5 mL serum separator tubes, centrifuged at 1100 _ g for 15 minutes within 1 hour of blood collection and the serum frozen at -70C until assayed. Specimens for C-peptide assays were drawn in chilled 5 mL sodium heparin/aprotinin tubes, centrifuged twice at 800 _ g for 10 minutes within 1 hour of blood collection, and the separated plasma frozen at -70C until assayed. Insulin and C-peptide were measured with standard chemiluminescence and immunochemiluminescence assays respectively. HOMA-_, HOMA-IR,14 and QUICKI15 were calculated at study entry and also postpartum, with conversion of glucose units to milligrams per deciliter in all equations, as follows: HOMA-_ = 20 _ fasting insulin (microinternational units per milliliter)/([0.055 _ fasting glucose (milligrams per deciliter)] - 3.5); HOMA-IR = fasting insulin (microinternational units per milliliter) _ fasting glucose (milligrams per deciliter)/405; QUICKI = 1/[log fasting insulin (microinternational units per milliliter) + log fasting glucose (milligrams per deciliter)].
The primary endpoint for this study was the incidence of impaired glucose tolerance in pregnancy, as defined by a glucose greater than 130 mg/dL 1 hour after a 50 g oral glucose load at study entry or between entry and delivery. Primary analyses categorized glucose tolerance as "always normal" (glucose testing normal at all study visits while pregnant) or "at least impaired glucose tolerance" (abnormal 1 hour glucose test with no or one abnormal value on 3 hour glucose tests; missing or incomplete 3-hour tests; or confirmed gestational diabetes). A sample size of 80 subjects per antiretroviral treatment group (160 subjects total) provided 80% power to detect a relative risk of 2.0 for the association between protease inhibitor exposure and impaired glucose tolerance using a 1-sided test, assuming 20% impaired glucose tolerance in the protease inhibitor-sparing group and 8.5% lost to follow-up or with uninterpretable results.
Groups were compared using Wilcoxon rank sum tests for continuous variables and Fisher exact or _2 tests for categorical variables. Logistic regression was performed to examine associations between subject characteristics, protease inhibitor use, and impaired glucose tolerance in pregnancy. All analyses were performed in SAS 9.1 (SAS Institute, Cary, NC).
Results
Of 161 subjects enrolled in the study, 3 never started the study protocol and 9 had missing data at the initial glucose test, leaving 149 (92%) evaluable subjects including 76 (51%) on protease inhibitors and 73 (49%) not on protease inhibitors. There were no significant differences in demographic, reproductive, virologic, or immunologic characteristics or body mass index between included and excluded subjects (data not shown).
As summarized in Table 1, there were no differences in demographic, reproductive, and virologic characteristics between protease inhibitor-including and protease inhibitor-sparing treatment groups. Fifty-five percent of subjects were black, 25% of Hispanic ethnicity, 15% white, and 5% from other racial groups. The median body mass index at study entry was 31 kg/m2. The most common protease inhibitors were nelfinavir (n = 44) and lopinavir/ritonavir (n = 25). The median duration of protease inhibitor use prior to entry was 140 days (interquartile range 69-757 days). All subjects in the protease inhibitor group continued on protease inhibitors until delivery or later. Four subjects not receiving protease inhibitors at study entry had received them in the past, and 1 subject started a protease inhibitor 7 days prior to delivery.
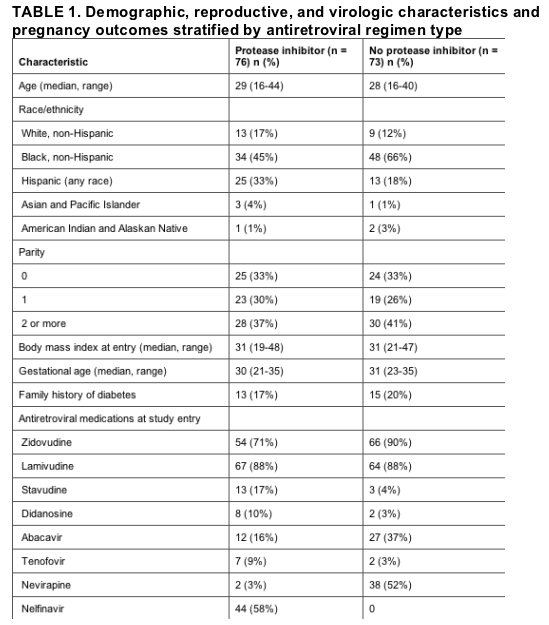
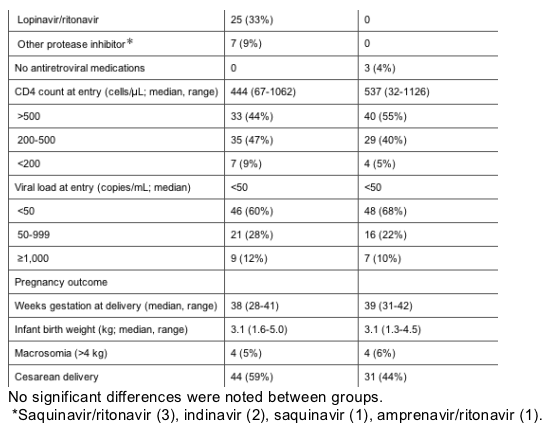
Delivery outcomes were similar between groups. There was 1 documented case of perinatal HIV transmission in the group not receiving protease inhibitors. Infant birthweight and neonatal macrosomia did not differ between treatment groups. Three infants had symptomatic neonatal hypoglycemia, 2 of whom were born to mothers receiving protease inhibitors and 1 born to a mother not receiving protease inhibitors. There were no concerning differences in toxicities or other infant outcomes between groups.
Table 2 summarizes glucose tolerance, fasting insulin, and indices of glucose metabolism at study entry. Only 62% of subjects had normal glucose tolerance in pregnancy; 29% had impaired glucose tolerance, and confirmed gestational diabetes occurred in 9% of subjects. Of the 57 subjects with impaired glucose tolerance, 47 (82%) were identified at study entry and 10 (18%) were detected as a result of follow-up testing during pregnancy. There were no significant differences in rates of impaired glucose tolerance or gestational diabetes between treatment groups. Duration of protease inhibitor therapy prior to enrollment was also not associated with glucose intolerance in pregnancy, either overall or when prolonged exposure was considered (more than 2 years). There were also no differences in fasting or 1 hour glucose, fasting insulin, HOMA-_, HOMA-IR, QUICKI, C-peptide, or glycosylated hemoglobin between antiretroviral treatment groups during pregnancy.
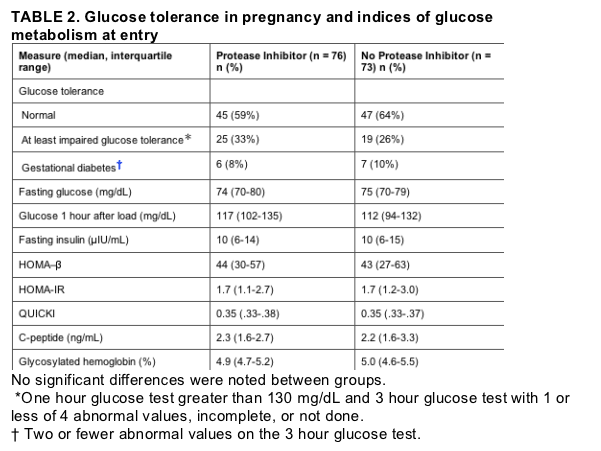
A multivariable analysis was performed to evaluate subject characteristics associated with glucose intolerance in pregnancy. Increased body mass index at study entry (P = .0024), Hispanic ethnicity (P = .012), and maternal age greater than 30 (P = .037) were all independently associated with impaired glucose tolerance with simultaneous adjustment for the other covariates. There were no interactions between race/ethnicity, body mass index, and glucose intolerance. Analysis with postpartum (instead of entry) body mass index did not change these findings. Protease inhibitor therapy was not associated with glucose intolerance in pregnancy in the multivariable analysis.
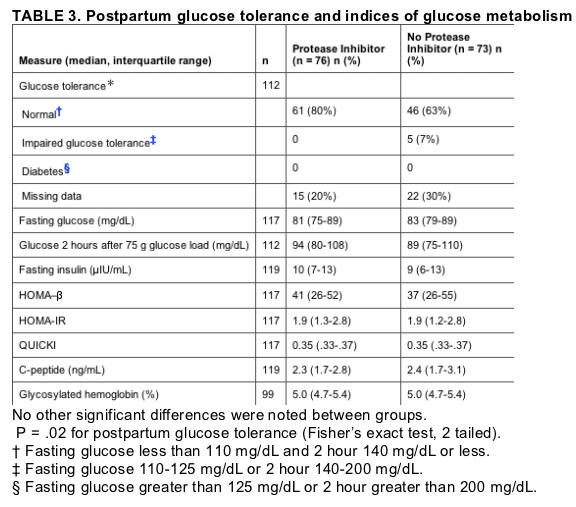
Only 61 of 76 subjects in the protease inhibitor group (80%) and 51 of 73 subjects in the protease inhibitor-sparing group (70%) had evaluable results for the 2 hour glucose tolerance test, so comparisons of postpartum glucose tolerance between groups were limited by missing data. No subject had diabetes. Of subjects with evaluable postpartum glucose testing, none receiving protease inhibitors and 5 of those not on protease inhibitors (7%) had impaired glucose tolerance (P = .02, Fisherfs exact 2-tailed test). There were no significant differences in postpartum fasting or 2 hour glucose, fasting insulin, indices of glucose metabolism, C-peptide, or glycosylated hemoglobin between antiretroviral treatment groups for those subjects with complete postpartum follow-up (Table 3).
Comment
This is the first prospective report with a primary objective to compare glucose tolerance between HIV-infected pregnant women on protease inhibitor-based therapy and those not receiving protease inhibitors. The finding of no difference in glucose intolerance between antiretroviral treatment groups agrees with several other recently published retrospective reports or secondary analyses of prospective cohorts.7, 8, 9 and 11 All subjects in this report had been on stable (or no) antiretroviral therapy for at least 8 weeks prior to study entry. Insulin resistance has been correlated with duration of protease inhibitor therapy, with 1 report finding that women with prolonged protease inhibitor use had an increased risk of glucose intolerance in pregnancy.10 However, in this study, no association was found between the duration of prior protease inhibitor exposure and glucose intolerance in pregnancy. There was also no evidence of an increase in subclinical insulin resistance as measured by fasting insulin, HOMA-IR, and QUICKI nor of alterations in insulin production as assessed by C-peptide and HOMA-_ between antiretroviral treatment groups. Thus, it appears unlikely that protease inhibitor-based antiretroviral therapy adversely affects insulin and glucose homeostasis during pregnancy.
One small prospective study examined glucose metabolism (area under the curve) during the 2 hours after a 75 g oral glucose load among HIV-infected pregnant women on protease inhibitor-based antiretroviral therapy or zidovudine monotherapy, compared with HIV-negative controls.19 All 3 groups of pregnant women had increasing glucose concentrations as pregnancy progressed, and the protease inhibitor group had a significantly higher glucose area under the curve in the late third trimester, compared with the HIV-negative group. There were no differences in rates of gestational diabetes or infant birthweight, and the clinical significance of this observation is uncertain.
Some reports have suggested that protease inhibitor therapy in pregnancy may increase the risk for premature birth,20 and 21 although others have found no association.10, 11 and 22 In this report, gestational age at delivery and infant birthweight did not differ between antiretroviral treatment groups. However, conclusions cannot be drawn from this cohort about the absolute rate of preterm birth, especially early preterm birth, because enrollment occurred up to 34 weeksf gestation, possibly limiting ascertainment of early preterm birth.
The observed overall 38% rate of impaired glucose tolerance and 9% rate of confirmed gestational diabetes in this cohort are considerably higher than the expected norm of 20-25% and 2-5% in the general obstetric population.12 The overall increased rate of impaired glucose tolerance in this cohort may be related to the relatively high body mass index and racial/ethnic composition of the subject population and may not be generalizable to all HIV-infected women. The risk factors for impaired glucose tolerance identified in this study have been observed in many other cohorts of HIV-negative pregnant women. It is possible, but not likely, that either HIV disease or antiretroviral medications other than protease inhibitors might have contributed to the increased rate of impaired glucose tolerance in this study. Inclusion of an HIV-negative comparison group could address this possibility in future studies.
This study used a more conservative (although accepted) definition of impaired glucose tolerance than many clinicians use in practice (130 vs 140 mg/dL 1 hour after a 50 g glucose load). Sixteen of 149 subjects (11%) had glucose test results between 131 and 149 mg/dL at study entry, and none of these women developed gestational diabetes. This study protocol also included serial glucose testing later in pregnancy than is the standard in clinical practice. Serial testing identified impaired glucose tolerance in 10 subjects with negative glucose testing at study entry (7%) and identified 2 additional cases of gestational diabetes (1 in each antiretroviral treatment group). Using the more standard cutoff of 140 mg/dL and including only glucose testing at entry would yield a 21% rate of impaired glucose tolerance in this cohort, which is comparable with the general population. However, the rate of gestational diabetes would be 7%, which is still higher than expected.
One limitation of this study was that missing or incomplete 3 hour glucose test data resulted in an inability to discriminate impaired glucose tolerance from gestational diabetes in 18 subjects. As such, the incidence of gestational diabetes in the cohort is probably underestimated. However, because the primary outcome was abnormal glucose tolerance (including gestational diabetes) and because missing data did not differ between antiretroviral treatment groups, an underestimate of gestational diabetes would be unlikely to mask a difference in glucose tolerance between groups.
Another limitation is that incomplete 2 hour glucose tests at the postpartum visit reduced the power to detect an effect of protease inhibitors on postpartum glucose tolerance and possibly introduced ascertainment bias, reducing the interpretability of the postpartum glucose tolerance results. Although we observed a marginally significant increase in impaired glucose tolerance among subjects not on protease inhibitors, missing data and the possibility of bias in testing make it unlikely that this finding represents a real difference in postpartum glucose tolerance between groups. Postpartum insulin and glucose homeostasis did not appear to differ between antiretroviral treatment groups among the subjects who had evaluable postpartum data.
One final caveat is that because this was not a randomized trial, it is possible that antiretroviral treatment selection could have introduced bias. Specifically, if women thought to be at increased risk for glucose intolerance were preferentially prescribed a protease inhibitor-sparing regimen, this could have obscured an association between protease inhibitors and glucose intolerance. Another potential bias might be preferential assignment to a protease inhibitor-sparing regimen for women with a prior history of gestational diabetes. The study did not collect information about prior gestational diabetes, so this possibility cannot be excluded.
In summary, in this prospective observational study, it does not appear that use of protease inhibitors in pregnancy is associated with abnormal glucose tolerance or with subclinical disruption in insulin and glucose homeostasis, despite the well-documented association between protease inhibitors and insulin resistance in nonpregnant adults. One might speculate that placentally mediated insulin resistance occurring in the context of normal pregnancy overwhelms any potential effect of protease inhibitors on insulin resistance in pregnancy. These findings, as well as the lack of adverse pregnancy or neonatal outcomes associated with protease inhibitor use in this report, are reassuring with regard to the widespread use of protease inhibitors during pregnancy for prevention of mother-to-child HIV transmission as well as for maternal health. The possibly increased rate of gestational diabetes observed in this study population is of concern, although it may be related to population characteristics such as high body mass index and race/ethnicity. If corroborated in other cohorts of HIV-infected pregnant women, further study of the relationships among HIV, antiretroviral therapy, body mass index, and gestational diabetes would be warranted.
|
|
| |
| |
|
|
|