| |
Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes FULL STUDY TEXT
|
| |
| |
Advanced Publication NEJM.
They will appear in the June 14, 2007 issue of the Journal.
Steven E. Nissen, M.D., and Kathy Wolski, M.P.H.
From the Cleveland Clinic, Cleveland.
"....Our data show that, as compared with placebo or with other antidiabetic regimens, treatment with rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that was of borderline significance.....These emerging findings raise an important question about the appropriateness of the current regulatory pathways for the development of drugs to treat diabetes. The FDA considers demonstration of a sustained reduction in blood glucose levels with an acceptable safety profile adequate for approval of antidiabetic agents. However, the ultimate value of antidiabetic therapy is the reduction of the complications of diabetes, not improvement in a laboratory measure of glycemic control.....Our study has important limitations (see Discussion below)... Despite these limitations, our data point to the urgent need for comprehensive evaluations to clarify the cardiovascular risks of rosiglitazone...."
ABSTRACT
Background Rosiglitazone is widely used to treat patients with type 2 diabetes mellitus, but its effect on cardiovascular morbidity and mortality has not been determined.
Methods We conducted searches of the published literature, the Web site of the Food and Drug Administration, and a clinical-trials registry maintained by the drug manufacturer (GlaxoSmithKline). Criteria for inclusion in our meta-analysis included a study duration of more than 24 weeks, the use of a randomized control group not receiving rosiglitazone, and the availability of outcome data for myocardial infarction and death from cardiovascular causes. Of 116 potentially relevant studies, 42 trials met the inclusion criteria. We tabulated all occurrences of myocardial infarction and death from cardiovascular causes.
Results Data were combined by means of a fixed-effects model. In the 42 trials, the mean age of the subjects was approximately 56 years, and the mean baseline glycated hemoglobin level was approximately 8.2%. In the rosiglitazone group, as compared with the control group, the odds ratio for myocardial infarction was 1.43 (95% confidence interval [CI], 1.03 to 1.98; P=0.03), and the odds ratio for death from cardiovascular causes was 1.64 (95% CI, 0.98 to 2.74; P=0.06).
Conclusions Rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that had borderline significance. Our study was limited by a lack of access to original source data, which would have enabled time-to-event analysis. Despite these limitations, patients and providers should consider the potential for serious adverse cardiovascular effects of treatment with rosiglitazone for type 2 diabetes.
Thiazolidinedione drugs are widely used to lower blood glucose levels in patients with type 2 diabetes mellitus. In the United States, three such agents have been introduced: troglitazone, which was removed from the market because of hepatotoxicity, and two currently available agents, rosiglitazone (Avandia, GlaxoSmithKline) and pioglitazone (Actos, Takeda). The thiazolidinediones are agonists for peroxisome-proliferator-activated receptor gamma (PPAR-y). PPAR-y receptors are ligand-activated nuclear transcription factors that modulate gene expression, lowering blood glucose primarily by increasing insulin sensitivity in peripheral tissues.1,2 Rosiglitazone was introduced in 1999 and is widely used as monotherapy or in fixed-dose combinations with either metformin (Avandamet, GlaxoSmithKline) or glimepiride (Avandaryl, GlaxoSmithKline).
The original approval of rosiglitazone was based on the ability of the drug to reduce blood glucose and glycated hemoglobin levels.3 Initial studies were not adequately powered to determine the effects of this agent on microvascular or macrovascular complications of diabetes, including cardiovascular morbidity and mortality.3 However, the effect of any antidiabetic therapy on cardiovascular outcomes is particularly important, because more than 65% of deaths in patients with diabetes are from cardiovascular causes.4 Therefore, we performed a meta-analysis of trials comparing rosiglitazone with placebo or active comparators to assess the effect of this agent on cardiovascular outcomes. The source material for this analysis consisted of publicly available data from the original registration package submitted to the Food and Drug Administration (FDA), another series of trials performed by the sponsor after approval, and two large, prospective, randomized trials designed to study additional indications for the drug.
Discussion
Our data show that, as compared with placebo or with other antidiabetic regimens, treatment with rosiglitazone was associated with a significant increase in the risk of myocardial infarction and with an increase in the risk of death from cardiovascular causes that was of borderline significance. The similar odds ratio for comparison with placebo suggests that the increased risk associated with rosiglitazone was not a function of the protective effects of active comparator drugs. However, these findings are based on limited access to trial results from publicly available sources, not on patient-level source data. Furthermore, results are based on a relatively small number of events, resulting in odds ratios that could be affected by small changes in the classification of events. Nonetheless, our findings are worrisome because of the high incidence of cardiovascular events in patients with diabetes.4 Because exposure of such patients to rosiglitazone is widespread, the public health impact of an increase in cardiovascular risk could be substantial if our data are borne out by further analysis and the results of larger controlled trials.
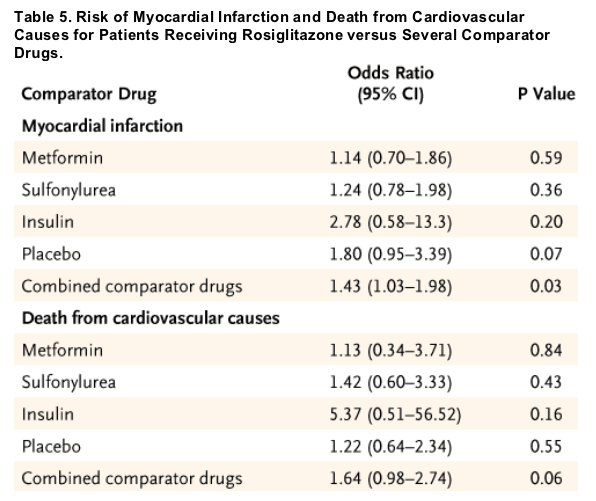
Although we did not have access to the source data to construct a composite outcome that included myocardial infarction or death from cardiovascular causes, the increase in the odds ratios for both of these end points suggests that observed adverse effects associated with rosiglitazone were probably not due to chance alone. This meta-analysis included a group of trials that were of relatively short duration (24 to 52 weeks). The odds ratio for these shorter-term trials was similar to the overall results of the meta-analysis. Thus, in susceptible patients, rosiglitazone therapy may be capable of provoking myocardial infarction or death from cardiovascular causes after relatively short-term exposure. In contrast, long-term therapies that improve cardiovascular outcomes, such as statins and antihypertensive drugs, often take several years to provide benefits. Notably, the estimates for the odds ratios for myocardial infarction and death from cardiovascular causes appear elevated for rosiglitazone in comparison with placebo or other commonly prescribed antidiabetic therapies (Table 5).
The mechanism for the apparent increase in myocardial infarction and death from cardiovascular causes associated with rosiglitazone remains uncertain. One potential contributing factor may be the adverse effect of the drug on serum lipids. The FDA-approved rosiglitazone product label reports a mean increase in low-density lipoprotein (LDL) cholesterol of 18.6% among patients treated for 26 weeks with an 8-mg daily dose, as compared with placebo.25 In observational studies and lipid-lowering trials, elevated levels of LDL cholesterol were associated with an increase in adverse cardiovascular outcomes. Thus, an increase in LDL cholesterol of the magnitude observed in the rosiglitazone group may have contributed to adverse cardiovascular outcomes, although the rapidity and magnitude of the apparent hazard was not consistent with an effect produced by lipid changes alone.
Several other properties of rosiglitazone may contribute to adverse cardiovascular outcomes. Rosiglitazone and other thiazolidinediones are known to precipitate congestive heart failure in susceptible patients.26 Congestive heart failure is a physiological state that is associated with an increased intravascular volume. Volume overload increases stress on the left ventricular wall, a factor that determines myocardial oxygen demand. In susceptible patients, an increase in myocardial oxygen demand could theoretically provoke ischemic events. The administration of thiazolidinediones, including rosiglitazone, also produces a modest reduction in the hemoglobin level.25 In susceptible patients, a reduced hemoglobin level may result in increased physiological stress, thereby provoking myocardial ischemia. A study of rosiglitazone that was conducted in rats reported an increase in the rate of death after experimentally induced myocardial infarction.27
Rosiglitazone is not the first PPAR agonist that has been reported to increase adverse cardiovascular events. Muraglitazar, an investigational dual PPAR-a and PPAR-y agonist, increased adverse cardiovascular events, including myocardial infarction, during phase 2 and 3 testing.28 After publication of an analysis of cardiovascular outcomes, muraglitazar was not approved by the FDA, and further development was subsequently halted by the manufacturer. Development programs for many other PPAR agonists have been terminated after evidence of toxicity emerged during preclinical studies or initial trials in humans. According to a former FDA official, more than 50 Investigational New Drug applications for novel PPARs have been filed, but no additional drugs have successfully reached the market in more than 6 years.29 In some cases, these drugs have failed because of evidence of direct myocardial toxicity in studies in animals,29 but few data on toxicity are available in the public domain because of the common industry practice of not publishing safety findings for failed products.
PPAR agonists such as rosiglitazone have very complex biologic effects, resulting from the activation or suppression of dozens of genes.30 The patterns of gene activation or suppression differ substantially among various PPAR agonists, even within closely related compounds. The biologic effects of the protein targets for most of the genes influenced by PPAR agonists remain largely unknown. Accordingly, many different and seemingly unrelated toxic effects have emerged during development of other PPAR agents.29 Some drugs have provoked multispecies, multi-organ system cancers; others have resulted in rhabdomyolysis or nephrotoxicity.29 Troglitazone was withdrawn from the market for rare, but sometimes fatal, liver toxicity. Accordingly, it must be assumed that a variety of unexpected toxic effects are possible when PPAR agonists are administered to patients.
The question as to whether the observed risks of rosiglitazone represent a "class effect" of thiazolidinediones must also be considered. Pioglitazone is a related agent also widely used to treat type 2 diabetes mellitus. However, unlike rosiglitazone, pioglitazone has been studied in a prospective, randomized trial of cardiovascular outcomes, called Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROACTIVE).31 The primary end point, a broad composite that included coronary and peripheral vascular events, showed a trend toward benefit from pioglitazone (hazard ratio, 0.90; P=0.095). A secondary end point consisting of myocardial infarction, stroke, and death from any cause showed a significant effect favoring pioglitazone (hazard ratio, 0.84; P=0.027). Notably, pioglitazone appears to have more favorable effects on lipids, particularly triglycerides, than does rosiglitazone.32
These emerging findings raise an important question about the appropriateness of the current regulatory pathways for the development of drugs to treat diabetes. The FDA considers demonstration of a sustained reduction in blood glucose levels with an acceptable safety profile adequate for approval of antidiabetic agents. However, the ultimate value of antidiabetic therapy is the reduction of the complications of diabetes, not improvement in a laboratory measure of glycemic control. Although reductions in blood glucose levels have been shown to reliably reduce microvascular complications of diabetes, the effect on macrovascular complications has proved to be unpredictable.33 After the failure of muraglitazar and the apparent increase in adverse cardiovascular outcomes with rosiglitazone, the use of blood glucose measurements as a surrogate end point in regulatory approval must be carefully reexamined.
Our study has important limitations. We pooled the results of a group of trials that were not originally intended to explore cardiovascular outcomes. Most trials did not centrally adjudicate cardiovascular outcomes, and the definitions of myocardial infarction were not available. Many of these trials were small and short-term, resulting in few adverse cardiovascular events or deaths. Accordingly, the confidence intervals for the odds ratios for myocardial infarction and death from cardiovascular causes are wide, resulting in considerable uncertainty about the magnitude of the observed hazard. Furthermore, we did not have access to original source data for any of these trials. Thus, we based the analysis on available data from publicly disclosed summaries of events. The lack of availability of source data did not allow the use of more statistically powerful time-to-event analysis. A meta-analysis is always considered less convincing than a large prospective trial designed to assess the outcome of interest. Although such a dedicated trial has not been completed for rosiglitazone, the ongoing Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trial may provide useful insights.34
Despite these limitations, our data point to the urgent need for comprehensive evaluations to clarify the cardiovascular risks of rosiglitazone. The manufacturer's public disclosure of summary results for rosiglitazone clinical trials is not sufficient to enable a robust assessment of cardiovascular risks. The manufacturer has all the source data for completed clinical trials and should make these data available to an external academic coordinating center for systematic analysis. The FDA also has access to study reports and other clinical-trial data not within the public domain. Further analyses of data available to the FDA and the manufacturer would enable a more robust assessment of the risks of this drug. Our data suggest a cardiovascular risk associated with the use of rosiglitazone. Until better precision of the estimates of the risks of this treatment on cardiovascular events can be delineated in patients with diabetes, patients and providers should give careful consideration to the risks and benefits of their overall treatment plans.
Dr. Nissen reports receiving research support to perform clinical trials through the Cleveland Clinic Cardiovascular Coordinating Center from Pfizer, AstraZeneca, Daiichi Sankyo, Roche, Takeda, Sanofi-Aventis, and Eli Lilly. Dr. Nissen consults for many pharmaceutical companies but requires them to donate all honoraria or consulting fees directly to charity so that he receives neither income nor a tax deduction. No other potential conflict of interest relevant to this article was reported.
Results
Baseline Characteristics
Table 2 reports the doses of rosiglitazone and comparator drugs, baseline demographic characteristics, study periods, and glycated hemoglobin levels or fasting blood glucose levels for patients enrolled in the trials. The patients were relatively young, averaging less than 57 years of age for both the rosiglitazone group and the control group. Overall, there was a moderate predominance of men. Diabetes control was relatively poor, with a mean baseline glycated hemoglobin level of approximately 8.2% for both study groups.
Myocardial Infarction and Death
Table 3 reports the myocardial infarction events and deaths from cardiovascular causes that were reported in the 42 clinical trials we reviewed. There were 86 myocardial infarctions in the rosiglitazone group and 72 in the control group. There were 39 deaths from cardiovascular causes in the rosiglitazone group and 22 in the control group. Table 4 lists the odds ratios, 95% confidence intervals, and P values for myocardial infarction and death from cardiovascular causes for the rosiglitazone group and the control group. The summary odds ratio for myocardial infarction was 1.43 in the rosiglitazone group (95% confidence interval [CI], 1.03 to 1.98; P=0.03). The odds ratio for death from cardiovascular causes in the rosiglitazone group, as compared with the control group, was 1.64 (95% CI, 0.98 to 2.74; P=0.06). Table 4 also lists odds ratios and 95% confidence intervals for the pooled group of trials that were smaller and of shorter duration; results for the DREAM and ADOPT studies are shown separately.
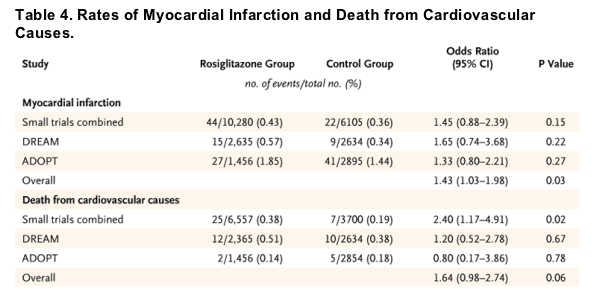
Table 5 lists odds ratios for myocardial infarction and death from cardiovascular causes associated with rosiglitazone for subgroups defined according to the comparator drug. Similar results were obtained when the analysis excluded trials with an active comparator group. The heterogeneity P values were 0.53 for myocardial infarction and 0.68 for death from cardiovascular causes across subgroups. As compared with placebo or other antidiabetic regimens, the estimated odds ratios in all cases were greater than 1.0, suggesting that observed adverse effects during rosiglitazone treatment were not unique to any specific comparator regimen.
In an analysis that was not prespecified, we also studied the effects of rosiglitazone on death from any cause. The odds ratio for death from any cause was 1.18 (95% CI, 0.89 to 1.55; P=0.24).
Methods
Analyzed Studies
Table 1 lists the 42 trials included in this meta-analysis. We screened 116 phase 2, 3, and 4 trials for inclusion. Of these, 48 trials met the predefined inclusion criteria of having a randomized comparator group, a similar duration of treatment in all groups, and more than 24 weeks of drug exposure. Six of the 48 trials did not report any myocardial infarctions or deaths from cardiovascular causes and therefore were not included in the analysis because the effect measure could not be calculated. Of the remaining 42 studies, 38 reported at least one myocardial infarction, and 22 reported at least one death from cardiovascular causes. In these trials, 15,560 patients were randomly assigned to regimens that included rosiglitazone, and 12,283 were assigned to comparator groups with regimens that did not include rosiglitazone.
Multiple groups of patients who received rosiglitazone within a single trial were pooled together, when applicable. The control group was defined as patients receiving any drug regimen other than rosiglitazone. The trials fall into three categories. One group includes five of the studies submitted to the FDA for the March 22, 1999, advisory board hearing that recommended approval of rosiglitazone. Group-level data from these five studies are available in publicly disclosed briefing documents archived on the FDA Web site.6 Data from these same trials are also reported in a summary fashion on a clinical-trial registry Web site maintained by the drug manufacturer, GlaxoSmithKline.5 Reports of four of these five trials were also published in peer-reviewed journals.7,8,9 In these five trials, 1967 patients were randomly assigned to receive rosiglitazone, and 793 patients were assigned to receive various comparator drugs (Table 1).
Other studies that we included in the meta-analysis were initially identified in the GlaxoSmithKline clinical-trial registry.5 As noted in Table 1, we included 35 studies in this category, 9 of which were published in peer-reviewed journals and 26 of which remain unpublished.10,11,12,13,14,15,16,17,18 Whenever possible, the results obtained on the GlaxoSmithKline Web site were cross-checked with the publication. In cases of disagreement between published and unpublished data, data derived from the manufacturer's Web site were used. In this group of 35 trials, 9502 patients were randomly assigned to receive rosiglitazone, and 5961 patients were assigned to receive various comparator drugs.
A third data source consisted of two large, recently published trials, the Diabetes Reduction Assessment with Ramipiril and Rosiglitazone Medication (DREAM) trial20 and the A Diabetes Outcome Prevention Trial (ADOPT) (ClinicalTrials.gov number, NCT00279045 [ClinicalTrials.gov] ).21 In the DREAM study, 2635 patients were randomly assigned to receive rosiglitazone and 2634 patients were assigned to receive placebo. The DREAM study was designed to determine whether rosiglitazone could prevent the development of type 2 diabetes in patients at high risk for this disorder. In the ADOPT trial, 1456 patients were randomly assigned to receive rosiglitazone and 2895 patients were assigned to receive either metformin or glyburide. The ADOPT study was designed to assess the durability of glycemic control with rosiglitazone therapy, as compared with therapy with metformin or glyburide.
Outcome Measures
We reviewed data summaries provided in the FDA review documents, the GlaxoSmithKline clinical-trial registry Web site, and published trial results and then abstracted from the adverse-event tabulations information on myocardial infarction and death from cardiovascular causes. With the exception of the DREAM study, the included trials did not describe adjudication of myocardial infarction or death from cardiovascular causes. Time-to-event data for cardiovascular events were not available in any of these trials, which precluded the calculation of hazard ratios. Because only summary data were available, it was not possible to discern whether the same patient had both events. Therefore, an outcome measure based on the composite of death or myocardial infarction could not be constructed. Accordingly, these two outcomes are reported separately.
Statistical Analysis
Many trials had few cardiovascular events, so the odds ratios and 95% confidence intervals were calculated with the use of the Peto method.22,23,24 Because all trials had similar durations of follow-up for all treatment groups, the use of odds ratios represents a valid approach to assessing the risk associated with the use of rosiglitazone. Trials in which patients had no adverse cardiovascular events in either group were excluded from analyses. All reported P values are two-sided. Statistical heterogeneity across the various trials was tested with the use of Cochran's Q statistic. A P value of more than the nominal level of 0.10 for the Q statistic indicated a lack of heterogeneity across trials, allowing for the use of a fixed-effects model. For additional analyses, the active comparator control groups were subgrouped into the following four classes for comparison with rosiglitazone: metformin, sulfonylurea, insulin, and placebo. Odds ratios and 95% confidence intervals were calculated for each subgroup with the use of methods similar to those used in the pooled analyses. Data were analyzed with the use of Comprehensive Meta-Analysis software, version 2.2 (Biostat).
|
|
| |
| |
|
|
|