| |
Eradication of HIV After 8 Years by HAART Started in Early Infection, NIH researchers hypothesize
|
| |
| |
Decay of the HIV Reservoir in Patients Receiving Antiretroviral Therapy for Extended Periods: Implications for Eradication of Virus
Journal of Infectious Diseases, June 2007
Tae-Wook Chun,1 J. Shawn Justement,1 Susan Moir,1 Claire W. Hallahan,2 Janine Maenza,3 James I. Mullins,3,4,5 Ann C. Collier,3 Lawrence Corey,3,5 and Anthony S. Fauci1
1Laboratory of Immunoregulation and 2Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland; Departments of 3Medicine, 4Microbiology, and 5Laboratory Medicine, University of Washington School of Medicine, Seattle
(See the editorial commentary by Margolis and Archin)
ABSTRACT:
The persistence of latently infected resting CD4+ T cells has been clearly demonstrated in human immunodeficiency virus (HIV)-infected individuals receiving effective antiviral therapy. However, estimates of the half-life of this viral reservoir have been quite divergent. We demonstrate clear evidence for decay of this HIV reservoir in patients who initiated antiviral therapy early in infection. The half-life of this latent viral reservoir was estimated to be 4.6 months. It is projected that it will take up to 7.7 years of continuous therapy to completely eliminate latently infected resting CD4+ T cells in infected individuals who initiate antiviral therapy early in HIV infection.
Despite the development of successful therapeutic strategies, it has not been possible to eradicate HIV in infected individuals, mainly because of the persistence of various viral reservoirs [1-5]. Among these reservoirs, a pool of latently infected cells in the resting CD4+ T cell compartment has been one of the most extensively studied to date and is considered to be a major impediment to HIV eradication [1-3]. Given that eradication of HIV is not possible as long as such infected cells persist, precise measurements of the half-life of this viral reservoir could shed light on the feasibility of completely eliminating HIV in patients receiving antiviral therapy, particularly as more potent therapeutic regimens become available, such as those that might contain an entry or integrase inhibitor; integration of provirus is critical for the establishment and replenishment of latently infected CD4+ T cells. However, previous studies addressing this subject have generated conflicting data (half-life ranging from 6.4 to 44.2 months) [6, 7]. To further delineate the decay rate of HIV and the possibility of the eradication of virus in a subset of patients who initiated antiviral therapy early in infection, we conducted longitudinal measurements of the frequency of resting CD4+ T cells carrying replication-competent virus from a previously identified cohort of patients in whom antiviral therapy was initiated soon after primary infection and in whom viral replication was extremely well controlled by therapy [8].
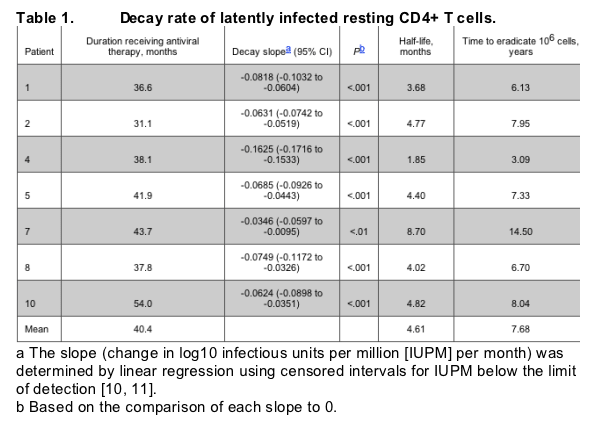
Patients, materials, and methods. Seven HIV-infected individuals who had received antiviral therapy for an average of 40.4 months (range, 31.1-54 months) (table 1) were studied. These individuals were participants in an earlier study [8]. The average time between the onset of symptoms of primary HIV infection and initiation of antiviral therapy was 2.7 months (range, 0.3-4.4 months). All patients received various antiviral regimens containing at least 1 protease inhibitor in addition to 2 reverse-transcriptase inhibitors and achieved maximal suppression of plasma viremia. Leukapheresis was conducted in accordance with protocols approved by the institutional review boards of the University of Washington, Seattle, and the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Multiple leukapheresis procedures followed by isolation of resting CD4+ T cells were conducted on each of the patients studied. Purified resting CD4+ T cells (up to 350 million cells) were subjected to a high-input coculture assay, and the frequency of cells carrying replication-competent HIV was calculated as described elsewhere [9].
Results.
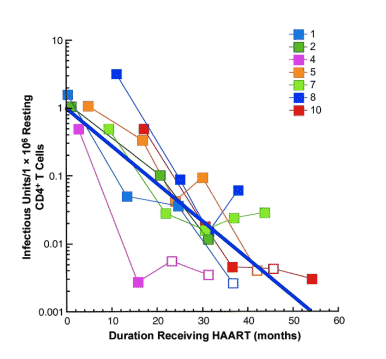
As shown in figure 1, decay of the latent viral reservoir was evident in all 7 individuals studied. Linear regression using censored intervals for infectious units per million resting CD4+ T cells below the level of detection with first-order kinetics was applied [10, 11]. On the basis of this analysis, the half-life of the latent reservoir was estimated to be 4.6 months (range, 1.9-8.7 months). With an assumption that 1 �~ 106 latently infected resting CD4+ T cells are present in an infected person, the projected time for complete elimination of HIV in the above cellular compartment would be 7.7 years (range, 3.1-14.5 years).
Figure 1. Decay of latently infected resting CD4+ T cells in patients receiving effective antiviral therapy. The data points for each individual are shown. The open symbols indicate values below the limit of detection. The solid line indicates the mean rate of decay.
Discussion. The persistence of HIV in the resting CD4+ T cell compartment has long been recognized as one of the major obstacles to achieving eradication of virus in infected individuals receiving effective antiviral therapy [1-3]. Previous studies have demonstrated that a long intrinsic half-life of the latent viral reservoir [7], along with low levels of residual viral replication in the periphery [5] and in lymphoid tissue [12], make eradication of virus all but impossible in infected individuals in whom antiviral therapy was initiated during the chronic phase of infection. However, the present study demonstrates that decay of the latent viral reservoir does occur in resting CD4+ T cells in patients in whom antiviral therapy was initiated during the early phase of HIV infection. The present study has potentially significant clinical implications, especially regarding the design of future therapeutic strategies aimed at eradicating HIV in infected individuals. Although ongoing HIV replication cannot be ruled out in infected individuals who began antiviral therapy early in HIV infection, it would be of considerable interest to begin to pursue evidence for the possibility of eradicating HIV in such individuals whose duration of therapy has reached the above figure. In this regard, recent studies have suggested that initiation of treatment shortly after seroconversion may facilitate decay of HIV in the CD4+ T cell compartment both in blood [13] and in gut-associated lymphoid tissues [14] in infected individuals receiving relatively short-term antiviral therapy. It is conceivable, if not likely, that eradication is not possible, at least with the currently available antiretroviral drug regimens; however, we are approaching the opportunity to address adequately this question based on projected half-lives of viral reservoirs in certain patients whose plasma viremia has been suppressed over extended periods of time. Of note, we have previously demonstrated that HIV rebounded on discontinuation of antiviral therapy in 2 of 2 chronically infected patients who were aviremic for 2-3 years and in whom no detectable virus could be demonstrated in CD4+ T cells in peripheral blood or lymph nodes [15]. However, these patients initiated antiviral therapy during the chronic phase of viral infection, and the duration of antiviral therapy was considerably less than 7.7 years, the projected time to eliminate HIV in the latent viral reservoir as demonstrated in the present study. Given that it has already been 10 years since antiviral therapy became available on a widespread basis, there are a number of patients who are or will soon fall into this category. A close monitoring of the size of viral reservoirs in patients who initiated antiviral therapy early in infection after successful control of HIV for longer than 8 years and who elect to discontinue therapy will be of considerable value in assessing the feasibility of eradication of HIV.
EDITORIAL
Eliminating Persistent HIV Infection: Getting to the End of the Rainbow
Journal of Infectious Diseases, June 2007
David M. Margolis1,2,3 and Nancie M. Archin1
Departments of 1Medicine, 2Microbiology and Immunology, and 3Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill
(See the brief report by Chun et al)
The medical community's response to the first 25 years of the HIV pandemic has been remarkable. It was recently estimated that the return on this investment has already yielded 3 million years of life saved in the United States alone [1]. However, the steps forward in the next 25 years will be, in some respects, even more difficult.
Antiretroviral therapy (ART) can return dying patients to health, but we are now challenged to maintain this benefit for the life of every patient, to avoid the toxicities of long-term chemotherapy, and to expand access to life-saving therapy across the world. To state the obvious, the development of safe microbicides and an effective prophylactic vaccine are the key requirements needed for the long-term control of the HIV pandemic. However, as illustrated poignantly by the recent halt of 2 trials of a promising microbicide, many obstacles are before us on the long path to these goals.
In this issue of Journal, the work of Chun et al. [2] speaks to another challenge for the next generation of HIV research: the eradication of established HIV infection. HIV infection is currently incurable because of at least 2 therapeutic shortcomings: the inability of current therapy to interrupt all active viral replication in an individual, and the inability to target persistent proviral infection.
In 1998, a collaboration between a group at the National Institute of Allergy and Infectious Diseases and the University of Washington, Seattle, determined the speed with which quiescent, latent infection of resting CD4+ T cells is established in acute or early HIV infection [3]. Ten patients were identified with symptoms consistent with early HIV infection, and ART was initiated 10-120 days after the onset of symptoms. Measurements of persistent, latent infection were performed after as little as a week of therapy and after up to 17 months of therapy, demonstrating that latent infection of resting CD4+ T cells was immediately established at a frequency of about 1 infected cell per million resting CD4+ T cells. The size of the "latent pool" was not substantially different in patients treated within 10 days of the onset of symptoms, which might be as little as 3 weeks after initial exposure to HIV, than in patients treated weeks later.
In their report in this issue of the Journal, Chun et al. provide follow-up on 7 of the members of the original cohort studied serially after 31-54 months of ART. Strikingly, the frequency of latent infection plummets in the first 12-24 months of ART, to levels 10-100-fold lower than typically seen: between 2.5 and 15 infected cells per billion resting CD4+ T cells. The suggestion that latent infection decays rapidly in the first 12-24 months of therapy during early infection is consistent with findings reported by Strain et al. in a cohort of patients treated within 6 months of seroconversion [4].
However, although Chun et al.'s study finds that the half-life of latently infected resting CD4+ T cells in this cohort is the shortest ever reported (4.6 months), a close examination of the data suggests that, at least in 4 of the patients studied, a second, slower phase of decay appears to exists. If so, the frequency of infection may not diminish to much less than 1 cell per billion after years of therapy. In this case, given the 110 billion resting CD4+ T cells carried by the average human [5], the few infected cells remaining may be enough to reignite infection. And, of course, the possibility exists that HIV may rarely persist in cells other than resting CD4+ T cells or that circulating resting cells somehow underrepresent infection in resting cells in the tissue.
As suggested by Chun et al., some patients who have undergone early ART for extended periods of time may choose to discontinue therapy. However, a conceptual challenge to interruption of ART in this setting is the evidence of ongoing low-level HIV replication in many patients [6-9]. Although HIV RNA is often undetectable in the cerebrospinal fluid of highly suppressed patients, ongoing low-level replication in the central nervous system despite ART is of concern [10]. Studies thus far of HIV replication in gut-associated lymphoid tissue suggest that ART may not be maximally effective at this primary site of lymphoid tissue replication [11]. It seems likely that improvements in current ART or alternate approaches are needed to target cellular or anatomic areas of potential pharmacologic inadequacy. As Chun et al. have done in the past, extensive study of cells and tissues in patients before and after treatment interruption would be critical to help us understand why infection was not extinguished, in the event that eradication is not observed.
So, what is the way forward from here? The treatment of acute HIV infection may set the stage for subsequent attempts at eradication. Programs developed using recent public health techniques to detect seronegative acute HIV infection [12, 13] are now being employed to help us better understand the factors that lead to transmission and to provide critical scientific insight for vaccine development and are being more widely considered as a public health measure to reduce the spread of HIV infection. These efforts may also result in a larger pool of patients treated during acute infection who may potentially benefit from future attempts at eradication of infection.
Targeted approaches to disrupt latent infection or speed the clearance of persistently infected cells have been proposed [14-17]. We found that intensified ART and valproate could deplete latent infection [18], but a recent report has not measured decay in resting CD4+ T cell infection in patients receiving standard ART who were prescribed valproate for clinical reasons [19]. When comparing these studies, it is important to recognize that there are subtle differences in the challenging assays used to quantify resting CD4+ T cell infection, resulting in differences in the reported frequency of infection in patient cohorts that are clinically similar [18-20]. However, the observation of greatest relevance would be one that irrefutably demonstrates a progressive and substantial depletion of persistent proviral infection, regardless of methodology. To achieve this, more potent ART and/or more potent antilatency therapies may be needed.
If the activity of such approaches can be validated in careful studies of stable patients during chronic infection, the clinical benefit of therapies for proviral infection might then be tested in patients treated during acute infection, for which lifelong therapy may not be clinically mandated. Immunotherapies such as vaccines to augment anti-HIV immunity and to prevent viral rebound once ART is discontinued might also be tested in such patients. However, given the great clinical success of ART and recent improvements in the simplicity and long-term tolerability of antiretrovirals, the bar for improving over current therapy is high. Whereas considerable toxicity is acceptable during chemotherapy for malignancies, therapies that attempt to eradicate HIV infection must avoid undue risk and toxicity [21].
Clearly, much work and many challenges lie ahead as we write the history of the second 25 years of AIDS. If we are able to continue to bring novel scientific insights to bear in clinically effective ways and to create access to these advances for the people who need them, the next chapter of the story will be even more impressive than the first.
|
|
| |
| |
|
|
|