| |
Effects of a Low-Glycemic Load vs Low-Fat Diet in Obese Young Adults
|
| |
| |
A Randomized Trial
Cara B. Ebbeling, PhD; Michael M. Leidig, RD; Henry A. Feldman, PhD; Margaret M. Lovesky, RD; David S. Ludwig, MD, PhD
JAMA. May 16, 2007;297:2092-2102.
"....The main finding of our study is that a simple measure of insulin secretion predicted weight and body fat loss on low-glycemic load and low-fat diets.... In conclusion, we found evidence for a diet-phenotype interaction involving insulin secretion. For obese individuals with high insulin concentration at 30 minutes during an oral glucose tolerance test, a low-glycemic load diet may promote more weight and body fat loss than a low-fat diet. Regardless of insulin secretion, a low-glycemic load diet has beneficial effects on concentrations of HDL cholesterol and triglycerides but not on LDL cholesterol."
ABSTRACT
Context The results of clinical trials involving diet in the treatment of obesity have been inconsistent, possibly due to inherent physiological differences among study participants.
Objective To determine whether insulin secretion affects weight loss with 2 popular diets.
Design, Setting, and Participants Randomized trial of obese young adults (aged 18-35 years; n = 73) conducted from September 2004 to December 2006 in Boston, Mass, and consisting of a 6-month intensive intervention period and a 12-month follow-up period. Serum insulin concentration at 30 minutes after a 75-g dose of oral glucose was determined at baseline as a measure of insulin secretion. Outcomes were assessed at 6, 12, and 18 months. Missing data were imputed conservatively.
Interventions A low-glycemic load (40% carbohydrate and 35% fat) vs low-fat (55% carbohydrate and 20% fat) diet.
Main Outcome Measures Body weight, body fat percentage determined by dual-energy x-ray absorptiometry, and cardiovascular disease risk factors.
Results Change in body weight and body fat percentage did not differ between the diet groups overall. However, insulin concentration at 30 minutes after a dose of oral glucose was an effect modifier (group x time x insulin concentration at 30 minutes: P = .02 for body weight and P = .01 for body fat percentage). For those with insulin concentration at 30 minutes above the median (57.5 μIU/mL; n = 28), the low-glycemic load diet produced a greater decrease in weight (-5.8 vs -1.2 kg; P = .004) and body fat percentage (-2.6% vs -0.9%; P = .03) than the low-fat diet at 18 months. There were no significant differences in these end points between diet groups for those with insulin concentration at 30 minutes below the median level (n = 28). Insulin concentration at 30 minutes after a dose of oral glucose was not a significant effect modifier for cardiovascular disease risk factors. In the full cohort, plasma high-density lipoprotein cholesterol and triglyceride concentrations improved more on the low-glycemic load diet, whereas low-density lipoprotein cholesterol concentration improved more on the low-fat diet.
Conclusions Variability in dietary weight loss trials may be partially attributable to differences in hormonal response. Reducing glycemic load may be especially important to achieve weight loss among individuals with high insulin secretion. Regardless of insulin secretion, a low-glycemic load diet has beneficial effects on high-density lipoprotein cholesterol and triglyceride concentrations but not on low-density lipoprotein cholesterol concentration.
COMMENT
The main finding of our study is that a simple measure of insulin secretion predicted weight and body fat loss on low-glycemic load and low-fat diets. For individuals with a low insulin concentration at 30 minutes after a 75-g dose of oral glucose, both diets produced comparable results. However, for those with a high insulin concentration at 30 minutes, the low-glycemic load diet was more efficacious for weight loss, consistent with an a priori hypothesis. Thus, phenotypic differences among individuals may explain some of the variability in individual outcomes within dietary weight-loss trials, and in mean outcomes among different trials.3, 5-11
The results of our outpatient study involving dietary counseling are consistent with 2 short-term feeding studies using hypocaloric diets in obese or overweight participants. Cornier et al38 studied 12 insulin-sensitive and 9 insulin-resistant women. Among those who were insulin-sensitive, weight loss was greater on a low-fat (60% carbohydrate, 20% fat) vs high-fat (40% carbohydrate, 40% fat) diet after 4 months (P<.01); among insulin-resistant individuals, weight loss was greater on the high-fat vs low-fat diet (P = .02). In a preliminary report, Pittas et al39 found that overweight participants with a high insulin concentration at 30 minutes on a low-glycemic load diet (40% carbohydrate, 30% fat; n = 8) lost more weight than those with a high insulin concentration at 30 minutes on a high-glycemic load diet (60% carbohydrate, 20% fat; n = 8; P = .05) after 6 months; no significant effect of diet on body weight was found among participants with a low insulin concentration at 30 minutes.
With regard to cardiovascular disease risk factors, HDL cholesterol and triglyceride concentrations improved more during the intensive intervention phase of the study for the full cohort on the low-glycemic load diet compared with the low-fat diet. (This difference persisted at 18 months for HDL cholesterol but not for triglyceride concentration.) These findings are consistent with previous research demonstrating benefits of low-carbohydrate or low-glycemic index diets40-41 with regard to components of the metabolic syndrome.42 Conversely, LDL cholesterol concentration (not a component of the metabolic syndrome) improved more on the low-fat diet. Although differential changes in LDL cholesterol concentration could relate to the baseline group difference, the lower saturated fat content of the low-fat diet provides a plausible explanation for this finding.43 When controlling for macronutrient content, some clinical trials show benefits of a low-glycemic index diet on LDL cholesterol concentration.11, 14 Therefore, we speculate that a low-glycemic load diet in which saturated fat is kept low (eg, by substituting monounsaturated or polyunsaturated fat from vegetable sources for saturated fat from animal sources) would have favorable effects on all 3 risk factors. Indeed, a recent epidemiological study involving 82 802 women over 20 years showed that a low-glycemic load diet high in vegetable fat and protein reduces risk of coronary heart disease.44
Several issues pertaining to study design warrant comment. Strengths of the study include a diverse cohort (with both sexes and several ethnic/racial groups) and relatively high follow-up rates of 90% at 6 months and 70% at 18 months. We analyzed data using the intention-to-treat principle, with conservative methods for imputing missing data. Also, we measured body composition using state-of-the-art dual-energy x-ray absorptiometry. Because this study used dietary counseling rather than meals prepared in a metabolic kitchen, these findings should have direct relevance to the management of obesity in routine clinical practice.
A methodological concern with most nutrition-related outpatient clinical trials is the possibility of bias because study participants consuming self-prepared diets and the study staff providing education and counseling generally cannot be masked to group assignment. However, we believe that this possibility has been minimized in our study for several reasons. First, considerable effort was made to maintain similar treatment intensity and treatment fidelity between groups. Second, process measures demonstrated that the intended changes in diet occurred in both groups, whereas protein and fiber, 2 potential confounders,45-46 did not differ between groups. Third, other process measures showed that physical activity and participant satisfaction also did not differ between groups. Moreover, dietitians delivered the interventions without knowing which individuals were in the low-concentration and high-concentration insulin strata at 30 minutes after a dose of oral glucose. Thus, the virtually identical weight loss for both diet groups in the stratum with low-insulin concentration provides further indication that the interventions were delivered without bias.
Limitations of this study include self-reporting for assessing diet and reliance on tabulated glycemic index values for quantifying glycemic load. Underreporting of dietary intake is a well-recognized phenomenon, common to all studies that aim to collect process data under free-living conditions, although adjusting other dietary variables for energy intake may partially correct for underreporting.47-48 With regard to tabulated glycemic index values,28 many were derived from studies conducted in countries where foods may differ in quality from those consumed in the United States. We used values derived from studies conducted in North America, when available. Moreover, we recognize that the outcomes observed in this study cannot be attributed exclusively to the effects of lowering dietary glycemic load. While we aimed to prescribe diets of similar protein and fiber content, other dietary factors (eg, energy density, palatability) may have differed between groups. Nevertheless, we note that this limitation would apply to all clinical trials of diet in the treatment of obesity in which participants consume self-prepared meals.
Statistical issues include the possibility of bias from use of imputed data, the modest sample size (particularly for analyses involving insulin concentration at 30 minutes at the later time points), and the possibility of "overfitting" too many covariates for the sample size. We do not believe that these concerns threaten the validity of the findings. Our conservative imputation strategy would tend to bias toward the null hypothesis. The SEs were based on pooled variance estimates from the full mixed-model analysis rather than potential underestimates from predominantly imputed values at the later time points. We included a random effect to take proper account of small-sample variability. Finally, the degrees of freedom expended on covariate adjustment amounted to a small fraction of the total number of data points, and their omission had negligible effect on the primary statistical tests.
In conclusion, we found evidence for a diet-phenotype interaction involving insulin secretion. For obese individuals with high insulin concentration at 30 minutes during an oral glucose tolerance test, a low-glycemic load diet may promote more weight and body fat loss than a low-fat diet. Regardless of insulin secretion, a low-glycemic load diet has beneficial effects on concentrations of HDL cholesterol and triglycerides but not on LDL cholesterol. Additional research is needed to examine these effects in other populations and to explore the mechanistic basis for the observed diet-phenotype interaction.
INTRODUCTION
With prevalence approaching one third of the population, obesity is among the most important medical problems in the United States1 and identification of effective dietary treatment has become a major public health priority.2 Three popular diets\low fat, low carbohydrate, and low glycemic load\have recently received much attention. However, clinical trials have produced inconsistent findings, with some suggesting that one diet is superior for weight loss3-8 and others indicating no difference between diets.9-11 This inconsistency may arise from methodological problems both within and between trials, such as different treatment intensity between groups, inadequate attention to treatment fidelity, variable nutrition education and dietary counseling strategies, and confounding by dietary and nondietary factors. An alternative explanation for this inconsistency relates to inherent physiological differences among study participants.
One physiological mechanism that might relate weight loss to dietary composition is individual differences in insulin secretion. Diets with a high glycemic load (the mathematical product of the glycemic index and the carbohydrate amount12) result in higher postprandial insulin concentration, calorie for calorie, than those with a low glycemic load.13 High postprandial insulin concentration has been postulated to decrease availability of metabolic fuels several hours after a meal, causing hunger and overeating.14 For this reason, we previously hypothesized that individuals with a high insulin response to glucose may be most sensitive to the effects of glycemic load.15 A translational study involving rodents supports this hypothesis: insulin concentration at 30 minutes after a dose of oral glucose predicted most of the variability in weight gain among animals consuming a high-glycemic index diet (R2 = 0.84; P<.001) but none of the variability among animals consuming a low-glycemic index diet (R2 = 0.003; P = .94).16
The purpose of this study was to determine whether insulin secretion affects body fat loss among obese individuals consuming self-prepared diets. Toward this end, we conducted an 18-month randomized controlled trial to compare the efficacy of a low-glycemic load/higher-fat diet with a low-fat/higher-glycemic load diet. To reduce the possibility of experimental bias, we aimed to keep treatment intensity, treatment fidelity, nutrition education and dietary counseling strategies, and physical activity prescription the same between the diet groups.
METHODS
Overview
Nutrition education and dietary counseling were provided to participants in both the low-glycemic load and low-fat diet groups. Prior to random assignment of participants to groups, a 75-g oral glucose tolerance test was conducted and serum was stored for later analysis of insulin. Body composition, plasma lipid levels, blood pressure, plasma glucose level, and serum insulin level were assessed at baseline and again at 6, 12, and 18 months. Body weight was tracked throughout the study. The institutional review board at Children's Hospital Boston approved the protocol. Each participant provided written informed consent before enrollment. Data were collected in Boston, Mass, between September 2004 and December 2006.
Participants
Participants were recruited using posted fliers, newspaper and Internet advertisements, and radio broadcasts that described the study as an opportunity for weight loss. Inclusion criteria included age between 18 and 35 years, body mass index (calculated as weight in kilograms divided by height in meters squared) of 30 and above, and medical clearance from a primary care provider. Exclusion criteria included body weight exceeding 140 kg, current smoking, recent adherence to a weight loss diet, use of medications that could affect study outcomes, and diabetes mellitus (fasting plasma glucose 126 mg/dL [7 mmol/L]) or any other major illness as assessed by a medical history and laboratory screening tests (blood urea nitrogen, creatinine, alanine transaminase, hematocrit). Seventy-three participants (15 males, 58 females) who met these criteria were enrolled in the study.
Enrolled participants were entered sequentially onto a list of random group assignments prepared in advance by the study statistician, with stratification by sex and ethnicity/race (based on participant self-report of non-Hispanic white or other). The sequence of random assignments was permuted within stratum in blocks of 2 and 4. To avoid any bias in assigning participants to diet groups, staff conducting recruitment and enrollment were masked to sequence. The study director assigned participants to groups. For completing study visits, participants were paid $50 at 6 months, $50 at 12 months, and $100 at 18 months.
Interventions
Low-Glycemic Load Diet. Participants were counseled to consume low-glycemic load foods (particularly nonstarchy vegetables, legumes, and temperate fruits) and to limit intake of high-glycemic load foods (such as refined grains, starchy vegetables, fruit juices, and sweets). Attention also was directed toward consuming sources of healthful fat including nuts, seeds, and oils. The target macronutrient composition was 40% of energy from carbohydrate, emphasizing low-glycemic index sources, 35% from fat, and 25% from protein. Participants were equipped with food-choice lists that delineated products into low-, moderate-, and high-glycemic load categories.17 Registered dietitians provided information during cooking demonstrations to encourage consumption of low-glycemic load foods and led interactive activities using food models to define appropriate serving sizes of high-glycemic load foods (eg, refined grain products, sweets).
Low-Fat Diet. Participants were counseled to consume low-fat grains, vegetables, fruits, and legumes and to limit intake of added fats, sweets, and high-fat snacks. The target macronutrient composition was 55% of energy from carbohydrate, 20% from fat, and 25% from protein. The intervention was not designed to maximize dietary glycemic index and glycemic load; rather, the aim was to prescribe a diet consistent with low-fat guidelines.18 Participants were equipped with food-choice lists that delineated products into low-, moderate-, and high-fat categories. Registered dietitians provided information during cooking demonstrations to encourage consumption of low-fat foods and led interactive activities using food models to define appropriate serving sizes of high-fat foods (eg, butter) and sweets.
Treatment Intensity. Diets were prescribed using an ad libitum approach, relying on intrinsic control of energy intake based on the presumption that these diets would decrease hunger, increase satiation and/or satiety, and therefore promote a negative energy balance. Proposed mechanisms for this presumption involve improved access to metabolic fuels on the low-glycemic load diet14, 19 and decreased energy density on the low-fat diet.20-23 An exchange system or a calorie-counting regimen was not used to impose an energy deficit, and participants did not receive any quantitative information regarding macronutrient targets. Rather, hunger and satiety cues were discussed and participants were advised as follows: "Eat when you are hungry, before you become famished. Stop eating when you are satisfied, before you become stuffed." Physical activity recommendations were consistent between groups and based on public health guidelines.24
The same intervention schedule, consisting of a 6-month intensive intervention period and a 12-month follow-up period, was implemented for both groups. There were 23 group workshops (1 hour each), 1 private counseling session (1 hour), and 5 motivational telephone calls (30 minutes each). Six of the group workshops were scheduled during the first 2 months of the intervention period, and the remaining workshops were held on a monthly basis thereafter. The private session occurred during the initial month, and a telephone call was scheduled for each of the subsequent 5 months of the intensive intervention period.
Nutrition Education and Dietary Counseling. Principles of nonformal adult education25 and participant-centered counseling26 were applied to promote adherence to the diets. As such, respectful consideration of participant perspectives, core values, life experiences, current circumstances, and available resources formed a foundation for education and counseling. The primary objective of the workshops was to foster knowledge and skills necessary to follow the respective diets, and the purpose of the telephone calls was to enhance motivation for translating knowledge and skills to changes in dietary behaviors. Dietitians were directive in negotiating goals with participants and empathetic when assisting them in overcoming adherence challenges. Participants were asked to keep one 3-day food diary prior to each workshop, particularly during the intensive intervention period, as a strategy for monitoring goal attainment. Dietitians prepared written feedback on submitted diaries, highlighting successes and providing advice for correcting deviations from diet prescriptions. To track progress, dietitians measured weight at each workshop using an electronic scale (model BWB-800, Tanita, Arlington Heights, Ill).
Treatment Fidelity. Dietitian adherence to the intervention protocols was conceptualized as treatment fidelity, a term encompassing integrity and differentiation.27 Integrity is the degree to which treatment is implemented according to established procedures, and differentiation is the extent to which interventions are distinct from one another. Several strategies were used to maximize treatment fidelity. First, group workshops were scripted and written educational materials were developed to ensure delivery of well-defined nutrition messages for each diet group; otherwise, the format of the workshops and quality of the materials were completely parallel to maintain equal treatment intensity. Second, flowcharts provided structure for the private session and motivational telephone calls and were used to foster dietitian adherence to a participant-centered counseling model, with adequate flexibility for addressing situations unique to each individual. Prompts for open-ended questions were included in the flowcharts to enhance dialogue. The private session and telephone calls were digitally recorded, such that the study director and lead dietitian could monitor deviations from the protocol and provide feedback to dietitians as necessary. The duration of the private session and each telephone call also was monitored as an indicator of integrity with regard to treatment intensity. Third, weekly staff meetings provided an opportunity for continued discussion on intervention delivery, particularly strategies for assisting individual participants without compromising differentiation between diets. Fourth, dietitians were given detailed guidelines for providing written feedback on food diaries to avoid unintended overlap in dietary advice between groups.
Process Evaluation
Participant adherence was evaluated based on attendance at group workshops and the private session, completion of motivational telephone calls, and self-reported dietary intake. In addition, data were obtained in regard to physical activity and participant satisfaction with the program.
Dietary and Physical Activity Recall Interviews. Three telephone-administered 24-hour recall interviews (2 weekdays and 1 weekend day) were conducted at baseline and again at 6, 12, and 18 months to assess diet and physical activity. These calls were separate from the motivational telephone calls that were part of the intervention. The recall interviews were unannounced so that the participant did not know the exact dates of the telephone calls in advance. The interviewer was masked to group assignment. Prior to the first interview, in-person training sessions were held on how to estimate food and beverage portion sizes and to rate the intensity of physical activity.
Dietary intake was assessed by a multiple-pass method using the Nutrition Data System for Research Software versions 5.0_35, 2005, and 2006 (Nutrition Coordinating Center, University of Minnesota, Minneapolis). The participant was prompted to list in sequence the foods and beverages consumed during the previous day, identify omissions in the initial list, and then provide details (eg, portion sizes, brand names) concerning each reported item. Intake was reviewed and confirmed at the end of the interview. Dietary variables of interest for this report include carbohydrate, total and saturated fat, protein, fiber, and energy intakes. Macronutrient (% of energy) and fiber (g/1000 kcal) intakes are reported relative to energy intake.
Dietary glycemic index and glycemic load for each day of self-reported intake were quantified as follows. First, glycemic index of individual carbohydrate-containing foods was assigned according to published values based on a glucose reference.28 When a published value was not available, the composition of the food was systematically evaluated to impute a value. The same glycemic index value was assigned to any given food every time that it was reported to avoid bias when evaluating differences between groups and changes over time. Second, the glycemic index for each food item was multiplied by the proportion of total carbohydrate contributed by the item to obtain a weighted glycemic index. Third, the daily glycemic index was calculated by summing the weighted values for each food item: (glycemic index for food item x proportion of total carbohydrate contributed by item). Fourth, glycemic load was calculated as the product of daily glycemic index and total carbohydrate intake and then adjusted for energy intake: (daily glycemic index/100 x grams of carbohydrate)/1000 kcal.
Following the dietary recall, the interviewer prompted the participant to recall physical activity and inactivity using a protocol modeled after established methods.29 The participant was asked to specify the activity performed most during respective 15-minute time blocks throughout the preceding day (12:00 AM-11:59 PM) and then to rate the relative intensity of each reported activity. A metabolic equivalent was assigned to each activity to calculate a physical activity factor (kcal/kg per hour). As points of reference, resting has a metabolic equivalent level of 1.0 and brisk walking has a level of 5.0.30
Participant Satisfaction With the Program. At the end of the 6-month intensive intervention period, participants responded to a series of questions regarding satisfaction, using 10-cm visual analog scales with appropriate verbal anchors. Questions addressed overall satisfaction with the diet and weight loss, ease of following the diet, and palatability of foods.
Study Outcomes
Data were collected by personnel who were masked to group assignment. Weight was measured using an electronic scale (Model 6702, Scale-Tronix, White Plains, NY) and height was measured using a wall-mounted stadiometer (Holtain Limited, Crymych, Wales). Body composition was assessed by dual-energy x-ray absorptiometry using Hologic instrumentation, models QDR 4500 and Discovery A (Hologic Inc, Bedford, Mass). Body fat percentage was calculated as the proportion of fat mass to total mass. Blood pressure was determined using an automated system (Model Pro 400, Dinamap, Tampa, Fla) with the participant sitting quietly. A blood sample was drawn by venipuncture, after a 12-hour overnight fast, and stored at -80C until assay for lipids, glucose, and insulin.
Assessment of Insulin Secretion
At a baseline assessment visit, each participant was given an oral glucose tolerance test using a standard 75-g dose of dextrose. Blood for determination of insulin concentration was obtained by indwelling venous catheter; serum from these samples was stored at -80C until assay. Insulin concentration 30 minutes after glucose consumption, the time point of particular interest, has been shown to be a good measure of insulin secretion in humans,31-33 and this time point was examined in our previous study in rodents.16 Dietitians involved in the interventions had no knowledge of insulin concentration at 30 minutes after the dose of oral glucose.
Laboratory Analyses
Plasma lipid concentrations were determined in a laboratory certified by the Lipid Standardization Program of the Centers for Disease Control and Prevention and the National Heart, Lung, and Blood Institute. Low-density lipoprotein (LDL) cholesterol level was measured by a homogeneous enzymatic assay (Genzyme Corp, Cambridge, Mass),34 and levels of high-density lipoprotein (HDL) cholesterol and triglycerides were measured using a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, Ind). Plasma glucose level was determined by an enzymatic colorimetric assay using a Hitachi 917 analyzer (Roche Diagnostics). Serum insulin level was quantified using a paramagnetic particle, chemiluminescence immunoassay (Access Immunoassay System, Beckman Coulter, Chaska, Minn).
Statistical Analysis
Flow of participants through the trial is presented in Figure 1. Data on insulin concentration at 30 minutes after a 75-g dose of oral glucose were not available for 17 of 73 participants who were randomly assigned to a diet group (8 in the low-glycemic load diet group and 9 in the low-fat diet group), primarily due to hemolysis when drawing timed blood samples. Hemolyzed samples were not analyzed in light of the well-documented effects of hemolysis on the accuracy of assays for quantifying insulin concentration.35 Moreover, there is no reason to believe that availability of blood samples for insulin analysis would influence responses to respective dietary interventions. Thus, effect modification by insulin concentration at 30 minutes was tested using a sample size of 56 participants (28 participants per diet group). In all analyses the intention-to-treat principle was used, classifying each participant in his/her randomly assigned diet group regardless of adherence or attendance.
Baseline demographic characteristics, body composition variables, and cardiovascular disease risk factors were compared between the diet groups using the Fisher exact test for categorical variables and the t test for continuous variables. The t test was corroborated by the Mann-Whitney-Wilcoxon 2-sample test in any case of mildly skewed distribution. Baseline characteristics were similarly compared between participants with high and low insulin concentration at 30 minutes, dividing those for whom insulin data were available into 2 strata: above the median (>57.5 μIU/mL) and below the median (57.5 μIU/mL). Treatment intensity and participant satisfaction measures were likewise compared using the t test.
Dietary intakes and physical activity level over the course of the trial were analyzed by mixed-model analysis of variance. The 4 time points (baseline and 6, 12, and 18 months) were represented by an arbitrary pattern (3 degrees of freedom) to avoid a global assumption of linearity or other functional form. Within-participant correlation was accounted for by a random effect (repeated measures with compound-symmetric covariance). Age, sex, cohort (3 waves of recruitment), and ethnicity/race (non-Hispanic white vs other) were included as covariates in all analyses. The covariate-adjusted means at each time point were used for graphical presentation. The group x time interaction term (3 degrees of freedom) provided a test of the hypothesis that the 2 dietary intervention groups did not differ across the study period. From parameters of the fitted model, a scalar contrast (1 degree of freedom) also was formed to compare the baseline measurement with the mean of all 3 postintervention measurements ([6 months + 12 months + 18 months]/3 - baseline). The difference in this contrast between the 2 diet groups served to summarize the group x time interaction.
Missing body composition measures, cardiovascular disease risk factors, and body weight data were imputed by a conservative strategy as follows. For intermittent missing values, the most recent prior measurement was imputed. For body fat percentage after dropout, either the last measurement obtained or the baseline value, whichever was greater, was imputed. For the cardiovascular disease risk factors after dropout, the last measurement obtained or the baseline value was imputed, whichever was least favorable (eg, higher for blood pressure, lower for HDL cholesterol). For body weight after dropout, an increase of 1 kg per year was imputed,36 starting from the last available measurement. All imputations were applied irrespective of group assignment.
The primary end point of the trial, body fat percentage, was analyzed by repeated-measures analysis of variance of the baseline and 6-, 12-, and 18-month measurements, as described above for dietary intakes, with the same covariates and covariance structure. Among the 56 participants with insulin response assessed at baseline, effect modification was tested by adding a dichotomous variable for insulin concentration at 30 minutes after a 75-g dose of oral glucose to the regression model (above or below median) and testing the 3-way interaction (group x time x insulin concentration at 30 minutes). To summarize and compare the changes overall and within high and low strata for insulin concentration at 30 minutes, scalar contrasts were formed from parameters of the fitted model, estimating the 6-month and 18-month changes (eg, [18-month - baseline] in the low-fat, high insulin concentration group). The 6-month point marked the end of the intensive intervention period and a time at which maximum weight loss often is observed in dietary interventions for obesity.37 The 18-month point marked the end of the follow-up period and a time when most long-term studies show substantial weight regain.37 Similar repeated-measures analyses were performed for lipids, blood pressure, and fasting glucose and insulin.
For body weight, measurements were obtained at the following time points: baseline and weeks 1, 2, 4, 5, 6, 10, 14, 17, 21, and 26; then every 4 weeks through week 74. For analysis of weight change, repeated-measures analysis of variance was used. An arbitrary pattern of variation for the 23 discrete time points was allowed from which contrasts of interest (eg, [6 months - baseline] in the low-glycemic load group - [6 months - baseline] in the low-fat group) and localized estimates of trend (eg, rate of weight loss over baseline to 26 weeks) could be formed. The same covariates and covariance structure were specified as described above. The overall diet effect was tested by the group x time interaction and the presence of effect modification (group x time x insulin concentration at 30 minutes). To summarize and compare the changes, scalar contrasts were formed from the fitted weight model representing net change at 18 months (eg, [18 months - baseline] in the low-fat, high insulin concentration group). Scalar contrasts also were constructed and compared for the linear trend in weight over each phase of the trial (baseline to 26 weeks, 26-50 weeks, and 50-74 weeks). To corroborate these results, effect modification was tested using baseline insulin concentration at 30 minutes after a dose of oral glucose as a continuous variable, log-transformed to reduce the influence of extreme observations. For graphical presentation, the raw weights were converted to changes from the participant's baseline measurements and repeated-measures analysis was performed on the resulting 22 discrete time points. The covariate-adjusted mean changes at each time point were displayed. All outcome variables were analyzed untransformed with the exception of plasma triglyceride concentration, which showed a marked skew in distribution. Therefore, log-transformed triglyceride values were used in a repeated-measures analysis and changes were presented as percentages (100% x [exp(change in log) - 1]).
The power assessment for the primary end point, body fat percentage, was based on a 2-sample t test with 36 participants per diet group and a 5% type I error rate. The mean (SD) baseline body fat percentage was 40.6 (5.6) in the sample of 73. Assuming a correlation of 0.9, the projected SD of change was (2 [1 - 0.9]) 1/2 x 5.6 = 2.5%. The sample size provided 80% power to detect an effect size of 0.67, or 0.67 x 2.5 = 1.7%. For effect modification by insulin concentration at 30 minutes after a dose of oral glucose in the available sample of 56, the effect size detectable with 80% power was 1.53, or 1.53 x 2.5 = 3.8%.
Computations were performed with SAS software version 9.01 (SAS Institute Inc, Cary, NC). Data are presented as mean (SE) unless otherwise indicated. Statistical significance was defined as P less than .05.
RESULTS
Baseline Measures
Baseline characteristics by diet groups and strata for insulin concentration at 30 minutes after a 75-g dose of oral glucose are presented in Table 1. There were no significant differences between diet groups, with the exception of LDL cholesterol concentration. Fasting insulin level and trunk fat were higher in the stratum with high insulin concentration (>57.5 μIU/mL) at 30 minutes after a dose of oral glucose than in the stratum with low insulin concentration (57.5 μIU/mL) at 30 minutes.
Process Measures
Changes in diet composition differed between groups as intended (Figure 2). The changes were expressed as the mean (95% confidence interval) of 6-, 12-, and 18-month intake compared with baseline (P value by analysis of variance). For the low-glycemic load group, glycemic index and carbohydrate intake decreased, producing a significant mean (SE) decrease in glycemic load (-19.8 [2.5] g/1000 kcal; P<.001). Total dietary fat increased (mean [SE], 3.0% [1.3%] of energy; P = .02) whereas saturated fat did not change (mean [SE], 0.5% [0.6%] of energy; P = .36). For the low-fat group, total fat intake decreased (mean [SE], -10.8% [1.3%] of energy; P<.001), as did saturated fat intake (mean [SE], -4.5% [0.6%] of energy; P<.001). Carbohydrate intake increased, producing an increase in glycemic load (mean [SE], 5.0 [2.5] g/1000 kcal; P = .05), even though glycemic index decreased slightly. Changes in consumption of protein and fiber and in physical activity did not differ between the groups.
Treatment intensity did not differ between diet groups (P>.40). On average, participants attended a mean (SE) of 13.4 (0.7) of the 23 scheduled workshops and completed 5.4 (0.2) of the 6 planned individual contacts with a registered dietitian. They provided food diaries on a mean (SE) of 5.6 (0.3) occasions during the 6-month intensive intervention period. Satisfaction with the program also did not differ between groups (Table 2). There were no adverse events during the intensive intervention period. Two adverse events occurred during the follow-up period, one unrelated to the protocol and the other possibly related (diagnosis of an eating disorder).
Body Weight and Body Fat Percentage
Change in body weight throughout the study is depicted in Figure 3. Weight loss did not differ between diet groups for the full cohort of 73 participants (P = .99). Among those for whom baseline data were available, insulin concentration at 30 minutes after a dose of oral glucose was a significant effect modifier (P = .02 for group x time x insulin concentration at 30 minutes). In the high-insulin concentration stratum, the low-glycemic load group lost weight more rapidly during the 6 months of intensive intervention (-1.0 vs -0.4 kg/mo; P<.001) and achieved greater overall weight loss at 18 months (-5.8 vs -1.2 kg; P = .004) compared with the low-fat group. Moreover, there was no evidence of weight regain after 6 months for participants in the stratum with high insulin concentration at 30 minutes who were assigned to the low-glycemic load group. Weight loss did not differ significantly between diet groups in the stratum with low insulin concentration at 30 minutes.
Treated as a continuous variable, insulin concentration at 30 minutes after a dose of oral glucose was related to weight change at 6 months in the low-glycemic load group (mean [SE], -1.2 [0.5] kg for each 2-fold increase in insulin concentration at 30 minutes; P = .02) but not in the low-fat group (mean [SE], 1.0 [0.7] kg for each 2-fold increase in insulin concentration at 30 minutes; P = .17). The net mean (SE) difference was -2.2 (0.9) kg for each 2-fold increase in insulin concentration at 30 minutes (P = .01).
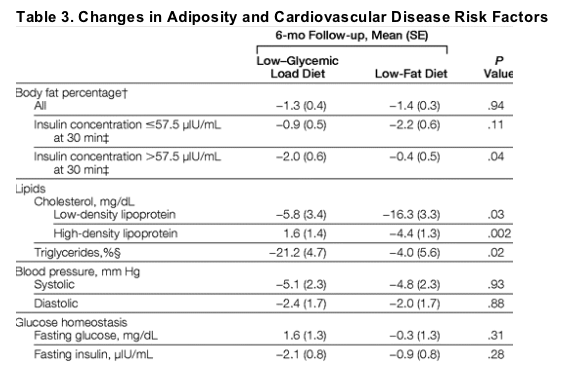
Change in body fat percentage also did not differ between diet groups for the full cohort over the course of the study (P = .81); although again, insulin concentration at 30 minutes was a significant effect modifier (P = .01). Body fat percentage decreased more in the low-glycemic load vs low-fat group, only among those in the stratum with high insulin concentration at 30 minutes (Table 3).
Cardiovascular Disease Risk Factors
Insulin concentration at 30 minutes after a dose of oral glucose was not a significant effect modifier for lipids, blood pressure, fasting glucose, and fasting insulin. Among the whole cohort, changes in LDL cholesterol, HDL cholesterol, and triglyceride concentrations differed significantly between diet groups. Changes in blood pressure, fasting glucose level, and fasting insulin level were not different between diet groups (Table 3).
|
|
| |
| |
|
|
|