| |
Prevalence of and Risk Factors for Pubic Lipoma (fat pad) Development in HIV-Infected Persons
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 45(1)1 May 2007pp 72-76
Guaraldi, Giovanni MD*; Orlando, Gabriella MD*; Squillace, Nicola MD*; Roverato, Alberto PhD*; De Fazio, Domenico MD; Vandelli, Marcella PhD; Nardini, Giulia PhD*; Beghetto, Barbara PhD*; De Paola, Maria PhD*; Esposito, Roberto MD*; Palella, Frank MD
"..In this report we describe the presence of PLs as a distinct manifestation of HIV-related lipoaccumulation that has not been previously reported. We believe that this condition represents a novel clinical manifestation of symmetric lipomatosis. A possible reason for its not having been previously described is that its clinical detection is most readily accomplished when patients are naked and standing. We observed PLs among a significant proportion (nearly 10%) of evaluated patients. Likewise, we identified a positive association between the presence of a PL and the simultaneous presence of dorsocervical fat pads (BHs), a known manifestation of HIV-associated symmetric lipomatosis.... the robustness of our findings, particularly a 9.4% prevalence rate of this never before described clinical entity in adult patients with lipodystrophy, underscore their significance and are unlikely to comprise a merely casual association with known HIV-associated body habitus changes. On the strength of these findings, we suggest that PLs be considered part of the HIV-associated lipodystrophy syndrome."
Abstract
Background: The natural history of HIV-associated body habitus changes is unclear. In this report, we describe a novel manifestation of HIV-associated lipoaccumulation.
Methods: We noted the presence of suprapubic fat pads (pubic lipomas [PLs]) in several patients with preexisting HIV-associated body habitus abnormalities. Subsequently, we evaluated the prevalence of and associated risk factors for development of PLs by undertaking an observational cross-sectional study among patients with known lipodystrophy who attended a metabolic clinic in northern Italy. Inclusion criteria were a physician-confirmed diagnosis of lipodystrophy according to the Multicenter AIDS Cohort Study definition and, for those affected with PL, a readily noticeable PL on physical examination.
Results:
We evaluated 582 patients with lipodystrophy: 214 female (36.7%) and 368 male (63.3%).
The overall PL prevalence was 9.4% (95% confidence interval [CI]: 7.2% to 12.1%; P < 0.0001).
PLs were more common among obese than nonobese individuals (34.5%, 95% CI: 17.9% to 5l.3% vs. 8%, 95% CI: 5.9% to 10.6%, respectively; P < 0.0001) and those with preexisting dorsocervical fat pads, commonly called buffalo humps (BHs) (18.5%, 95% CI: 12.7% to 25.4% vs. 6.1%, 95% CI: 4.03% to 8.83%, respectively, P < 0.0001; relative risk = 3.02, 95% CI: 1.84% to 4.96%, P < 0.0001).
The PL prevalence in the nonobese HIV-infected population (body mass index [BMI] <30, n = 550) was 8.0% (95% CI: 5.9% to 10.6%; P < 0.0001).
Logistic regression analyses identified the following factors as associated with a greater likelihood for PL: BMI >30 (_ = 0.18, SE = 0.04; P < 0.001), female gender (_ = 1.06, SE = 0.31; P < 0.001), and shorter duration of HIV infection (_ = -0.005, SE = 0.003; P = 0.04). We used a chain graph model to evaluate risk factors for BH and PL simultaneously. A nonnull interaction between these entities was evident, and this association seemed to be independent of factors positively associated with both (BMI and gender).
Conclusions:
PL is a newly recognized manifestation of HIV-associated lipoaccumulation that is more likely to occur among those with coexisting dorsocervical fat pads, suggesting the possibility of a common pathogenesis between the 2 entities. Likewise, PLs are more common among women, obese individuals, and those with a shorter duration of HIV infection. We suggest that PL should be considered part of the HIV-associated lipodystrophy syndrome.
HIV-associated morphologic abnormalities include body habitus changes characterized by subcutaneous lipoatrophy (subcutaneous fat loss on the trunk, extremities, and buttocks and facial wasting) and lipoaccumulation in subcutaneous areas (dorsocervical fat pads, often referred to as buffalo humps [BHs], as well as other symmetric and asymmetric lipomatoses and breast enlargement) and centrally (increases in visceral intra-abdominal fat giving rise to abdominal obesity).
At present, no universally accepted clinical definition of lipodystrophy exists. Most studies of HIV-infected individuals have defined regional fat gain or loss subjectively by patient self-report and/or confirmation on examination.1-19 Some studies have used objective tools in their definitions, including anthropometry3-5,7,12,20 or imaging findings.3-5,7,15,17 An objective case definition has been proposed but is seldom used in the clinical setting.21
Medical literature suggests that the etiology of HIV-associated body habitus changes is multifactorial. Although variable in manifestation, lipoaccumulation and lipoatrophy have been associated with risk factors that fall into 3 main categories: host factors, stage of HIV infection, and antiretroviral drug exposure.1,4,10,17,20,22,23 In particular, associations exist between lipoatrophy and cumulative exposure to thymidine analogue nucleoside reverse transcriptase inhibitors (TA-NRTIs) and between lipoaccumulation, including dorsocervical fat pad (BH) emergence, and use of first-generation protease inhibitors (PIs).24
The object of this report is to describe a novel clinical manifestation of lipoaccumulation, herein first reported on, in a large population of HIV-infected persons with known lipodystrophy who attended a metabolic clinic. Included are descriptions of its prevalence and associated factors.
RESULTS
We analyzed data from 582 adult patients (all white), including 214 female (36.7%) and 368 male (63.3%) patients, all of whom had been evaluated for the presence of a PL.
Fifty-four patients met the criteria for a moderate or severe PL. Twenty-eight (51.8%) were female and 26 (48.2%) were male. Of 528 evaluated patients who did not have a moderate or severe PL, 196 (37.2%) were female and 332 (62.8%) were male.
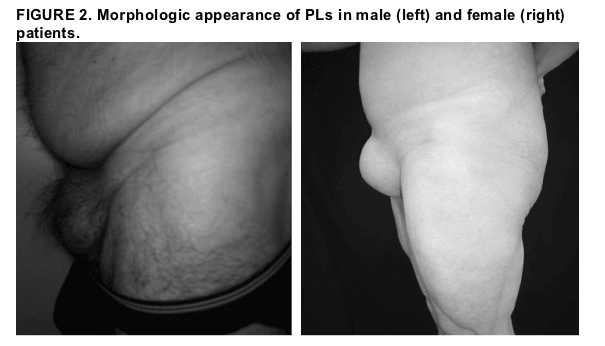
Figure 2 includes photographs demonstrating the morphologic appearance of PLs in an affected male patient and an affected female patient.
The overall prevalence of PLs in the study group was 9.4%. Table 1 profiles comparative demographic, metabolic, and morphologic data as well as HIV history and treatment data among those who did versus did not demonstrate a moderate to severe PL.
We observed that those with a PL were more likely than those without a PL to be obese (16.4% vs. 3.6%, respectively; P < 0.0006), to have a dorsocervical fat pad, or BH (50.8% vs. 22.2%, respectively; P < 0.001), to have multiple lipomatosis (22.4% vs. 8.5%, respectively; P = 0.002), and to demonstrate lipoaccumulation in other body areas simultaneously (61.5% vs. 36.6%; P < 0.0001) and were less likely to demonstrate lipoatrophy (13.8% vs. 45.6%, respectively; P < 0.0001).
To explore PL prevalence in nonobese lipodystrophic patients, 32 individuals who had a BMI >30 were excluded from the analysis. The prevalence of PLs in the nonobese HIV-infected population was 8.0% (95% confidence interval [CI]: 5.9% to 10.6%; P < 0.0001). An increased likelihood of a PL was strongly associated with increasing BMI, even in BMI ranges not suggestive of obesity (see Fig. 3A; P = 0.015 for those with a BMI ≥30 vs. <30).
The prevalence of PLs in people with a prominent dorsocervical fat pad, or BH, was 18.5% (95% CI: 4.03% to 8.83%; P < 0.0001) versus 6.1% in those without a BH (95% CI: 12.7% to 25.4%; P < 0.0001) with a relative risk of 3.02 (95% CI: 1.84% to 4.96%; P < 0.0001). Again, an increased likelihood of a BH was strongly associated with increasing BMI, even in BMI ranges not suggestive of obesity (see Fig. 3B).
Patients with PLs demonstrated lower median scores evaluating esthetic self-satisfaction with their body image when compared with those without PLs, as measured using a visual analog scale (VAS) assessment (see Table 1).
We used logistic regression models to evaluate for factors associated with BHs and PLs individually. A BMI >30 (_ = 0.24, SE = 0.03; P < 0.001), female gender (_ = 0.68, SE = 0.21; P < 0.01), protease inhibitor (PI) use (_ = 0.059, SE = 0.003; P < 0.05), lamivudine use (P < 0.0001), stavudine use (P < 0.0004), indinavir use (P < 0.04), ritonavir use (P < 0.001), and saquinavir use (P < 0.03) were each positively associated with a BH. A BMI >30 (_ = 0.18, SE = 0.04; P < 0.001), female gender (_ = 1.06, SE = 0.31; P < 0.001), and shorter duration of HIV infection (_ = -0.005, SE = 0.003; P = 0.04) were the only variables positively associated with the presence of a PL.
To evaluate for factors potentially linked to BHs and PLs, we used a chain graph model, as described by Edwards,26 in which BH and PL represent a single joint outcome and the covariates are gender, BMI, and PI use. By applying a stepwise backward elimination search procedure, we obtained the model represented in Figure 1, which provided a good fit of the data (deviance = 61.23 with 69 df; P = 0.74). According to this model, PI use was independent of BMI and gender. Furthermore, although increased BMI and female gender were positively associated with BHs and PLs, PI use was associated with BHs but not with PLs. The presence of a connecting line between BH and PL is to be interpreted as the existence of a nonnull interaction between these 2 outcomes that cannot be explained merely by their shared association with BMI and gender, both of which exert a simultaneous effect on the presence of BHs and PLs that is accounted for by the model.
DISCUSSION
In this report we describe the presence of PLs as a distinct manifestation of HIV-related lipoaccumulation that has not been previously reported. We believe that this condition represents a novel clinical manifestation of symmetric lipomatosis. A possible reason for its not having been previously described is that its clinical detection is most readily accomplished when patients are naked and standing. We observed PLs among a significant proportion (nearly 10%) of evaluated patients. Likewise, we identified a positive association between the presence of a PL and the simultaneous presence of dorsocervical fat pads (BHs), a known manifestation of HIV-associated symmetric lipomatosis.
Furthermore, in logistic regression analyses, we noted an increased likelihood of PLs among women, among those with a shorter duration of HIV infection, and among those who were obese. Although BMI was consistently the factor most strongly associated with PLs across all BMI ranges, it is important to note that, even after excluding those with obesity, we discerned a substantial overall PL prevalence of 8.0%. It would thus seem unlikely that a PL is merely diffuse fat accumulation in the context of obesity.
Although we identified risk factors common to PLs and BHs (BMI and female gender), it is interesting to note that exposure to PIs emerged as a risk factor for BHs, as previously reported in the literature,23 but not for PLs.
HIV-associated body habitus changes among women, in contrast to men, more frequently include manifestations of lipoaccumulation.27,28 Our finding of an association of PLs with female gender is consistent with these observations.
Our data indicate that those with PLs were less pleased with their physical appearance. In particular, having a PL was perceived among our patients as a barrier to normal sexual functioning.
Reasons for the apparent association between PLs and shorter duration of HIV infection are unclear. Because lipoatrophy, the most common form of HIV-associated body habitus alteration, has been associated with more advanced HIV infection (and, by implication, with longer duration of HIV infection) and with the use of TA-NRTIs, the development of a PL in those with longer duration of HIV, more advanced disease, or greater TA-NRTI exposure may somehow be suppressed or at least attenuated. We were not able to explore this issue thoroughly in our analyses, however.
Other limitations to our report are clear. First, we lack an objective quantifiable clinical definition of PL. PLs were diagnosed by physical inspection much in the same way as dorsocervical fat pads (BHs). The presence of a nonnull interaction between BH and PL in the chain graph model (which cannot be explained by BMI or gender) could be interpreted to imply the possibility of an as yet unknown pathogenic mechanism that is operative in PL and BH development. Clearly, these issues can only be evaluated adequately and systematically in a large prospective cohort of HIV-infected persons in which objective measurements, including anthropomorphic imaging assessments with ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) are routinely undertaken to assess and verify abnormalities noted on clinical examination. Likewise, we did not have a readily available HIV-seronegative control group for comparison.
Despite these limitations, we believe that the robustness of our findings, particularly a 9.4% prevalence rate of this never before described clinical entity in adult patients with lipodystrophy, underscore their significance and are unlikely to comprise a merely casual association with known HIV-associated body habitus changes. On the strength of these findings, we suggest that PLs be considered part of the HIV-associated lipodystrophy syndrome.
|
|
| |
| |
|
|
|