| |
Ischemic Heart Disease in HIV-Infected and Uninfected Individuals: A Population-Based Cohort Study
|
| |
| |
Clinical Infectious Diseases June 2007
Niels Obel,1 Henrik F. Thomsen,4 Gitte Kronborg,2 Carsten S. Larsen,5 Per R. Hildebrandt,3 Henrik T. Sorensen,4,6 and Jan Gerstoft1
1Department of Infectious Diseases, Rigshospitalet, 2Department of Infectious Diseases, Hvidovre, and 3Department of Cardiology, Frederiksberg, Copenhagen University Hospital, Copenhagen, and Departments of 4Clinical Epidemiology and 5Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark; and 6Department of Epidemiology, Boston University, Boston, Massachusetts
"....we observed an increased risk of ischemic heart disease in HIV-infected patients immediately after HAART initiation. The risk increase was moderate and of the same order as that introduced by smoking 1-4 cigarettes per day [26], and it was less than that observed in patients with insulin-dependent diabetes [27]. We found no evidence of an increase in the relative risk over time, which suggests that HAART does not have an aggressive atherogenic effect...."
ABSTRACT
Background. There are concerns about highly active antiretroviral therapy (HAART) causing a progressive increase in the risk of ischemic heart disease. We examined this issue in a nationwide cohort study of patients with human immunodeficiency virus (HIV) infection and a population-based control group.
Methods. We determined the rate of first hospitalization for ischemic heart disease in all Danish patients with HIV infection (3953 patients) from 1 January 1995 through 31 December 2004 and compared this rate with that for 373,856 subjects in a population-based control group. Data on first hospitalization for ischemic heart disease and comorbidity were obtained from the Danish National Hospital Registry for all study participants. We used Cox's regression to compute the hospitalization rate ratio as an estimate of relative risk, adjusting for comorbidity.
Results. Although the difference was not statistically significant, patients with HIV infection who had not initiated HAART were slightly more likely to be hospitalized for the first time with ischemic heart disease than were control subjects (adjusted relative risk, 1.39; 95% confidence interval, 0.81-2.33). After HAART initiation, the risk increase became substantially higher (adjusted relative risk, 2.12; 95% confidence interval, 1.62-2.76), but the relative risk did not further increase in the initial 8 years of HAART.
Conclusions. Compared with the general population, HIV-infected patients receiving HAART have an increased risk of ischemic heart disease, but the relative risk is stable up to 8 years after treatment initiation.
Concerns have been raised that HIV-infected patients treated with HAART have a progressively increasing risk of ischemic heart disease because of dyslipidemia induced by the therapy [1, 2]. Two studies have examined the impact of HAART on intima media thickness, measured by ultrasonography, as a marker for ischemic heart disease, with inconsistent findings [3, 4]. Cohort studies of ischemic heart disease in HIV-infected patients also have had conflicting results [5-11]. The limitations of these studies included the use of different data sources to ascertain cases of ischemic heart disease in the patients with HIV infection and in the control group or the absence of control subjects from the general population. The latter may be of particular importance, because the diagnosis of ischemic heart disease has evolved during the study periods. To overcome these methodological shortcomings, we conducted a cohort study of ischemic heart disease in Danish patients with HIV infection and control subjects from the general population using 3 nationwide registries: the Danish HIV Cohort Study, the Danish Civil Registration System, and the Danish National Hospital Registry [12-14]. The study was designed to examine whether HIV-infected patients receiving HAART have an elevated relative risk of a first hospitalization for ischemic heart disease, compared with the general population. If that prediction proved to be correct, the study further aimed to establish whether this disparity in relative risk increased with duration of HAART.
METHODS
Setting
As of 1 January 2005, Denmark had a population of 5,400,000; the estimated prevalence of HIV infection in the adult population is 0.07% [13]. Denmark's tax-funded health care system provides antiretroviral treatment free of charge to all HIV-positive residents. Treatment of HIV infection is provided in only 8 specialized medical centers, where patients are seen on an outpatient basis at intended intervals of 12 weeks. During our study period, national criteria for HAART initiation were presence of an HIV-related disease, acute HIV infection, pregnancy, CD4+ cell count <300 cells/L, and, until 2001, a plasma HIV RNA load >100,000 copies/mL. Structured treatment interruptions have generally not been recommended in Denmark.
Study Population and Data Collection
HIV cohort. The Danish HIV Cohort Study, which has been described elsewhere, includes all HIV-infected patients treated in the 8 specialized centers in Denmark from 1 January 1995 through 31 December 2004 [13]. The cohort includes 4252 Danish residents (as recorded in the Danish Civil Registration System) who received a diagnosis of HIV infection before 1 January 2005 and were >16 years of age at the time of diagnosis.
In our study, the index date was defined as the HIV infection diagnosis date for all cohort members except those who received a diagnosis before 1 January 1995; for the latter patients, the index date was set at 1 January 1995. The study included all 3953 Danish patients who (1) lived in Denmark on the index date and (2) were not hospitalized with ischemic heart disease prior to this date. HAART was defined as a treatment regimen of at least 3 antiretroviral drugs that included a nonnucleoside reverse-transcriptase inhibitor, a protease inhibitor, and/or abacavir, or a treatment regimen with a combination of a nonnucleoside reverse-transcriptase inhibitor and a boosted protease inhibitor.
General population control cohort. The Danish Civil Registration System, which has stored information on all Danish residents since 1968, was used to identify control subjects from the general population for the study [12]. For each person, it records a 10-digit unique identification number, date of birth, sex, residence location, dates of immigration or emigration, and date of death. We aimed to identify 99 control subjects for each HIV-infected patient, matched by sex, age (month and year of birth), and municipality of residence. Because of the shortage of eligible control subjects in some municipalities, we identified 94.5 control subjects per patient with HIV infection (a total of 373,856 control subjects). We also extracted data on death and emigration for patients with HIV infection and the control subjects from the Danish Civil Registration System.
Hospitalization with Ischemic Heart Disease and Comorbidities
Hospitalization data for all study subjects were obtained from the Danish National Hospital Registry, established in 1977 [14]. This registry contains records of all discharge diagnoses (coded according to the International Classification of Diseases, 8th revision, until the end of 1993, and the International Classification of Diseases, 10th revision, codes thereafter) and procedure codes for patients treated in Danish hospitals. We defined the study outcome, "the first hospitalization for verified ischemic heart disease," as a first-time discharge diagnosis of myocardial infarction (codes 410.09 or 410.99 in International Classification of Diseases, 8th revision; codes I21.0 to I21.9 in International Classification of Diseases, 10th revision) or a first-time coronary artery bypass and percutaneous coronary intervention (procedure codes KFNA00 to KNFG96). From the Danish National Hospital Registry, we also extracted data on angina pectoris, sudden death from heart disease, heart diseases other than the study outcome, and comorbidities known to be risk factors for ischemic heart disease (diabetes, alcoholism, hypertension, liver disease, and kidney disease).
Statistical Analysis
We constructed Kaplan Meier curves for time until first hospitalization for ischemic heart disease according to HAART status. Follow-up ceased on the earliest of the following events: date of death, emigration, loss to follow-up, first hospitalization with ischemic heart disease, or 1 January 2005. The period from the index date until HAART initiation or end of follow-up, whichever came first, was considered to be the non-HAART period. The time following HAART initiation was considered the HAART period, even if treatment interruptions occurred.
We used stratified Cox's regression to compute rate ratios of the first hospitalization with ischemic heart disease as estimates of relative risk. We assessed the proportional hazards assumption with plots and tests that were based on smoothed scaled Schoenfeld residuals. Each comorbidity diagnosis was included in the regression model as a design variable. Separate analyses were performed for the non-HAART and HAART periods. For the HAART period, subanalyses were performed for 2 time intervals: <90 days of HAART and >90 days of HAART. We also computed rates of first hospitalization with ischemic heart disease for 2-year periods and up to 8 years after HAART initiation.
Because accuracy of coding may differ by discharge diagnosis, we performed several sensitivity analyses to examine how changes in definition affected the findings. In one analysis, we included angina pectoris and sudden cardiac death in the definition of first hospitalization for verified ischemic heart disease. In another analysis, we defined the outcome as only the first-time discharge diagnosis of myocardial infarction.
Data analysis was performed using SPSS software, version 12.0 (Norusis; SPSS), and R Development Core Team software (R Foundation for Statistical Computing). The study was approved by the Danish Data Protection Agency.
RESULTS
The study cohort included 3953 HIV-infected patients and 373,856 control subjects (table 1). Patients and control subjects were well-matched in terms of age at index date, sex, emigration, and loss to follow-up. During the non-HAART period, the risk of first hospitalization for ischemic heart disease was slightly higher in patients with HIV infection than in control subjects (adjusted relative risk, 1.39; 95% CI, 0.82-2.36) (figure 1 and table 2). During the HAART period, the risk increase was substantially higher (adjusted relative risk, 2.12; 95% CI, 1.62 to 2.76) (figure 2 and table 2). Examination of the smoothed scaled Schoenfeld residuals indicated a higher relative risk of first hospitalization for ischemic heart disease in the first 90 days after HAART initiation (data not shown). In a separate analysis of this time period, the adjusted relative risk for a first ischemic heart disease hospitalization was 7.44 (95% CI, 3.35-16.5). This is considerably higher than during the rest of the HAART period, for which the adjusted relative risk was 1.92 (95% CI, 1.45-2.55) (table 2).
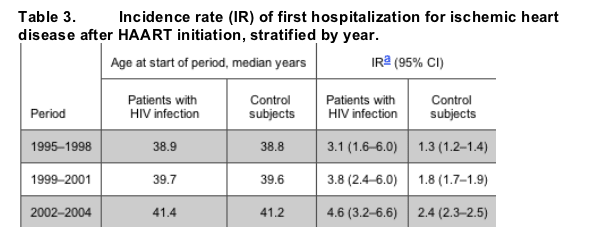
Within the 2-year intervals following HAART initiation, the rates of first hospitalization for ischemic heart disease increased in the HIV-infected individuals (0-2-year period, 3.82 cases per 1000 person-years of observation [PYR] [95% CI, 2.44-5.99 cases per 1000 PYR]; 6-8-year period, 6.51 cases per 1000 PYR [95% CI, 3.50-12.1 cases per 1000 PYR]). However, compared with the control subjects, the relative risk for patients treated with HAART did not increase during the 8-year period after HAART initiation (figure 3). From the period 1995-1998 to the period 2002-2004, the incidence of first hospitalization for ischemic heart disease in control subjects increased from 1.3 cases per 1000 PYR to 2.3 cases per 1000 PYR, and in the same interval, the median age of control subjects increased from 38.8 years to 41.2 years. These 2 parameters increased in the HAART-exposed population, as well (table 3).
Among patients with a CD4+ cell count >200 cells/uL at HAART initiation, relative risk, compared with control subjects, was 1.80 (95% CI, 1.17-2.78), and among patients with a CD4+ cell count <200 cells/uL, relative risk was 2.28 (95% CI, 1.63-3.19). Patients initiating HAART with a viral load of <105 HIV RNA copies/mL had a relative risk of ischemic heart disease of 1.52 (95% CI, 0.98-2.37), compared with a relative risk of 2.52 (95% CI, 1.69-3.78) among patients with a viral load >105 HIV RNA copies/mL. For patients initiating HAART within 1 year after receiving a diagnosis of HIV infection, relative risk of ischemic heart disease was 2.38 (95% CI, 1.56-3.64), and among patients whose HAART treatment was delayed longer than 1 year following receipt of a diagnosis of HIV infection, relative risk was 1.90 (95% CI, 1.36-2.65). During the 30 days after the first hospitalization for ischemic heart disease, 5 (8.8%) of the patients with HIV infection died, compared with 107 (3.8%) of the control subjects.
When first hospitalization for ischemic heart disease was defined only as a discharge diagnosis of myocardial infarction, the relative risk estimate remained unchanged; for the non-HAART period, the relative risk was 1.37 (95% CI, 0.75-2.48), and for the HAART period, the relative risk was 2.29 (95% CI, 1.69-3.09). Including angina pectoris and sudden cardiac death in the definition of the first hospitalization for ischemic heart disease also had little impact on relative risk estimates; for the non-HAART period, the relative risk was 1.47 (95% CI, 0.88-2.45), and for the HAART period, the relative risk was 2.05 (95% CI, 1.57-2.67). Excluding nonwhite individuals from the analysis had almost no effect on the estimates; for the non-HAART period, the relative risk was 1.34 (95% CI, 0.77-2.32), and for the HAART period, the relative risk was 1.99 (95% CI, 1.51-2.62).
DISCUSSION
We found that, after initiation of HAART, the risk of ischemic heart disease was higher among HIV-infected patients than among control subjects. However, we observed no progressive increase in the relative risk in the 8-year period following HAART initiation.
In this study, we used a truly population-based control cohort and were able to achieve a longer follow-up period than that achieved by earlier studies. Comparison with a population-based control group allowed us to account for potential bias introduced from differences in age and calendar time. Importantly, we used the same source of data to ascertain outcome for all study subjects. We are aware of no other study with a similar design addressing the important question of HAART-related cardiac events.
We relied on registry-based discharge diagnoses to ascertain the almost 5000 outcome events in this study. Although discharge diagnoses in general may not be entirely accurate, the registration of myocardial infarction, coronary bypass surgery, and percutaneous coronary intervention have been shown to be highly valid [14, 15]. Because the clinical criteria for myocardial infarction and indications for invasive procedures were revised during the study period [16], the increase in risk of first hospitalization for ischemic heart disease likely reflects the increase in age among the study population and changes in diagnostic and treatment practices related to ischemic heart disease. Although we missed patients who died before hospitalization for ischemic heart disease, we assume that rates of prehospitalization death from this cause are not likely to differ between patients and control subjects. Therefore, potential underreporting of ischemic heart disease culminating in death before hospitalization did not bias our relative risk estimates.
An important study limitation is lack of data on certain risk factors for ischemic heart disease, such as smoking, which is presumed to be more frequent among HIV-infected patients than among the general population [17]. Failure to account for smoking may have led us to overestimate the relative risk of ischemic heart disease associated with HAART. Also, we cannot exclude the possibility that the general population receives more-optimal treatment for potential risk factors for ischemic heart disease. However, we find it unlikely that major changes in general risk-factor profiles occurred suddenly at the time of HAART initiation. We therefore expect that estimates of changes in relative risk over time are robust.
In contrast to our study, the Data Collection on Adverse Events of Anti-HIV Drugs (D.A.D.) study reported that HAART caused a progressive increase in the risk of ischemic heart disease, with relative risk increasing by 26% for each year of HAART [7]. The study used a prospective cohort design, but it did not include follow-up of all patients from the time of HAART initiation, had a shorter follow-up period, and did not include an HIV-negative control population. The latter may be crucial, because we observed a substantial increase over calendar time in the frequency of first hospitalization with ischemic heart disease in the control subjects, as well as in the HIV-infected population (table 3). In recent years clinicians have focused on the risk of ischemic heart disease, and increased guidance from blood lipid tests has expanded the use of statins and antiretroviral regimens with more-favorable lipid profiles. These changes in treatment strategies may potentially diminish a putative increase in the relative risk of ischemic heart disease. However, the D.A.D. study found that the risk profile for ischemic heart disease has worsened over time [18]. In our study, blood lipids were not measured systematically among patients with HIV infection, and we did not have access to data on the lipid profiles of the control subjects. Although blood lipids are likely to be on the causal pathway, absence of information prohibited us from estimating any potential residual effect of HAART.
The sudden increase in the risk of ischemic heart disease after HAART initiation, observed both in our study and in the D.A.D. study, parallels findings of increased cholesterol and triglyceride blood levels in a number of other studies [19, 20]. However, the magnitude and the timing of the changes indicate that other factors are also involved in the disease process. A more gradual increase in the risk would be expected if lipid accumulation in the atherosclerotic plaques were the main risk factor for ischemic heart disease after HAART initiation. Other mechanisms, such as inflammation or changes in platelet or endothelial function, must be considered as potential explanations for the sudden expansion and instability of the atherosclerotic plaques. It is noteworthy that, during the initial months after HAART initiation, an ill-defined syndrome of paradoxical immune reconstitution occurs in some patients as a response to opportunistic pathogens [21]. This syndrome is associated with low nadir CD4+ cell counts and rapid increase in CD4+ cell counts [22, 23].
We observed a higher risk of first ischemic heart disease hospitalization during the initial months after HAART initiation and found a larger relative risk among patients who initiated HAART with a higher viral load and lower CD4+ cell count, suggesting that immune reconstitution may partly explain the excess risk after HAART initiation. However, we cannot rule out the possibility that the association between HAART initiation and excess risk of first hospitalization with ischemic heart disease is caused partly by a measurement bias: patients with HIV infection have more frequent contacts with the health care system and, therefore, are more likely to have ischemic heart disease detected than are members of the general population [24]. Given the abrupt increase in the risk of ischemic heart disease following HAART initiation, body composition changes and derived metabolic changes are unlikely to be causal factors in this process, because these changes usually occur after years of antiretroviral therapy. Partly in accordance with our observations, the Strategies for Management of Antiretroviral Therapy study found that starting and stopping HAART might be related to an increased risk of cardiovascular incidents [25].
In conclusion, we observed an increased risk of ischemic heart disease in HIV-infected patients immediately after HAART initiation. The risk increase was moderate and of the same order as that introduced by smoking 1-4 cigarettes per day [26], and it was less than that observed in patients with insulin-dependent diabetes [27]. We found no evidence of an increase in the relative risk over time, which suggests that HAART does not have an aggressive atherogenic effect. The pathogenetic basis for the increase needs to be clarified to optimize prophylaxis. Furthermore, it should be established whether risk of ischemic heart disease is linked to specific drug classes, as a recent study has suggested [28].
|
|
| |
| |
|
|
|