| |
GSK Defends Rosiglitazone in Letter to The Lancet: "Cardiovascular safety of rosiglitazone"
|
| |
| |
Letter to The Lancet
Ronald L Krall, MD
Chief Medical Officer, GlaxoSmithKline, King of Prussia, PA 19406, USA
Published Online, May 30, 2007DOI:10.1016/S0140-6736(07)60824-1
In response to your Editorial (published online May 23)1 regarding the study in the New England Journal of Medicine by Steve Nissen and Kathy Wolski,2 I would like to provide further perspective. Nissen and Wolski estimate a 43% increase in myocardial infarction associated with rosiglitazone. In an associated Editorial, Bruce Psaty and Curt Furberg3 allege that if their estimate is valid there has been a failure of drug use and approval.
GlaxoSmithKline did similar meta-analyses in 2005 and 20064 and found hazard ratios in the same direction as Nissen and Wolski. However, all these results are highly dependent on the methods used and the studies included, given the small number of events reported. For example, the actual number of myocardial infarctions in the Nissen and Wolski meta-analysis yields a very low frequency of events (0.6%), and the absolute difference in rates of myocardial infarctions between rosiglitazone and controls is less than 0.1%.
These observations support a view expressed by Nissen and Wolski them-selves: "a meta-analysis is always considered less convincing than a large prospective trial designed to assess the outcome of interest." There are three such trials on which we can rely, two of which have completed, and one, which although still ongoing, has undergone an informative interim analysis.
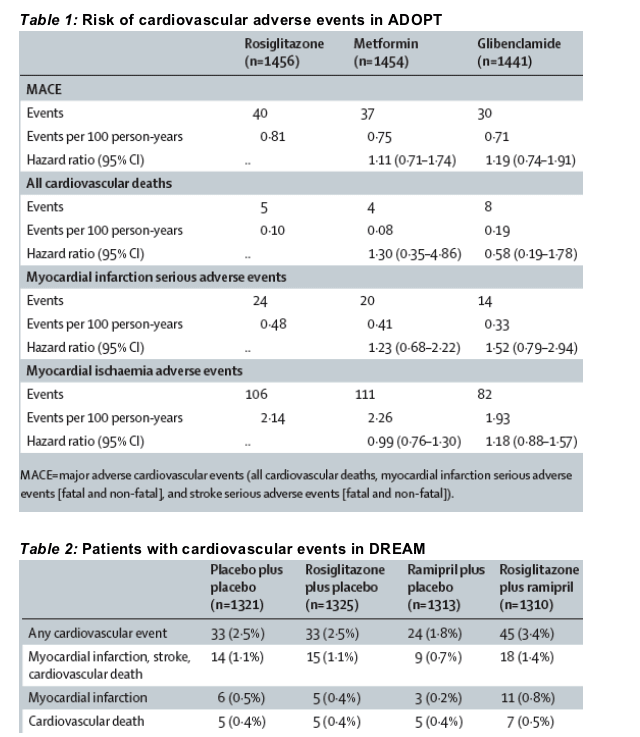
The first trial, ADOPT (A Diabetes Outcome Progression Trial),5 was a 4-6 year study of glycaemic durability in 4360 people recently diagnosed with type 2 diabetes. Patients were randomly assigned to monotherapy with rosiglitazone, metformin, or glibenclamide for a median of 4a0 years. Since publication of the primary paper, GlaxoSmithKline has further analysed the ADOPT database, examining all major adverse cardio-vascular events (table 1, see below). Our analysis, which adjusted for medication exposure, found that such events were rare in this population and that all treatments were comparable. Hazard ratios for the comparisons between rosiglitazone and the other standard oral antidiabetic agents, metformin and glibenclamide, varied from 0.58 to 1.52 and 95% CIs for all comparisons included unity.
Data from the DREAM (Diabetes Reduction Assessment with ramipril and rosiglitazone Medication)6 trial provide a similar picture of the cardiovascular profile for rosiglitazone. Briefly, DREAM assessed whether long-term treatment with rosiglitazone (or ramipril) can reduce the risk of type 2 diabetes in 5269 patients with impaired glucose tolerance or impaired fasting glucose. The trial had a randomised, double-blind, 2x2 factorial design, in which patients were randomised to rosiglitazone or placebo and to ramipril or placebo. In their initial publication, the DREAM study investigators reported no significant difference between the rosiglitazone-containing groups (rosiglitazone plus placebo and rosiglitazone plus ramipril) and the placebo groups (ramipril plus placebo and placebo plus placebo) in their secondary composite endpoint of cardiovascular events (myocardial infarction, stroke, cardiovascular deaths, confirmed heart failure, new angina, and revascularisation procedures). A cell-level intention-to-treat analysis of the final DREAM database by GlaxoSmithKline found that similar numbers of patients on rosiglitazone, ramipril, and placebo had cardiovascular events (table 2). The increased numbers of events in the rosiglitazone plus ramipril group of the study is currently unexplained.
The most compelling evidence comes from RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes),7 an open-label, 6-year cardiovascular outcomes trial (with prospectively defined cardiovascular endpoints) in 4458 patients that started in 2000. The independent data safety monitoring board for RECORD recently reviewed an interim analysis of unblinded cardiovascular endpoints and confirmed that the trial should continue (manuscript in preparation).
Other cardiovascular outcomes trials, such as the 2368-patient Bypass Angio-plasty Revascularisation Investigation Type 2 Diabetes trial (BARI 2D)8 and the 10 251-patient ACCORD (Action to Control Cardiovascular Risk in Diabetes) study, will further inform the cardiovascular safety profile of rosiglitazone. Their data safety monitoring boards have also confirmed that those studies should continue.
Finally, confirmation of the observations made in ADOPT, DREAM, RECORD, and the other cardiovascular outcome trials can be found by examining the usual care of patients with type 2 diabetes. In 2006, Glaxo-SmithKline commissioned a balanced-cohort observational study in a managed-care database of 33,363 patients who began oral antidiabetic treatment between 2000 and 2004. The study, which assessed a composite cardiovascular endpoint of hospital admissions for myocardial infarction, coronary revascularisation, or both, compared rosiglitazone, metformin, or sulfonylurea as mono-therapy, dual-therapy combinations, and insulin combinations. The incidence of the composite cardiovascular endpoint was 1.75 events per 100 patient-years for the rosiglitazone-containing regimen and 1.76 events per 100 patient-years for the non-rosiglitazone-containing regimen (hazard ratio 0.93, 95% CI 0.80-1.10).
We believe that these studies provide clear evidence of the cardiovascular safety of rosiglitazone and that the estimates of cardiovascular morbidity from the meta-analyses completed to date are not robust. The drug use and approval system is working. We should stay the course and allow ongoing trials to provide their definitive answers.
REFERENCES
1 The Lancet. Rosiglitazone: seeking a balanced perspective. Lancet 2007; published online May 23. DOI:10.1016/S0140-6736(07)60787-9.
2 Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; published online May 21. DOI:10.1056/NEJMoa072761.
3 Psaty BM, Furberg CD. Rosiglitazone and cardiovascular risk. N Engl J Med 2007; published online May 21. DOI:10.1056/NEJMe078099.
4 GlaxoSmithKline. Clinical trials register: rosiglitazone studies. Study no ZM2005/0018/01 and study no HM2006/00497/00 / WEUSRTP866. http://ctr.gsk.co.uk/Summary/rosiglitazone/studylist.asp (accessed May 25, 2007).
5 Kahn SE, Haffner SM, Heise MA, et al, for the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006; 355: 2427-43.
6 DREAM (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 2006; 368: 1096-105.
7 Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocol. Diabetologia 2005; 48: 1726-35.
8 Magee MF, Isley WL, for the BARI 2D Trial Investigators. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97 (12A): 20G-30G.
|
|
| |
| |
|
|
|