| |
Food Omega-3 Fatty Acids Lower Blood Pressure & Should Impact Heart Disease/Dyslipidemia, study finds
|
| |
| |
Food Omega-3 Fatty Acid Intake of Individuals (Total, Linolenic Acid, Long-Chain) and Their Blood Pressure INTERMAP Study
Hypertension June 7. 2007;50:1-7
Hirotsugu Ueshima, Jeremiah Stamler, Paul Elliott, Queenie Chan, Ian J. Brown,
Mercedes R. Carnethon, Martha L. Daviglus, Ka He, Alicia Moag-Stahlberg, Beatriz L. Rodriguez, Lyn M. Steffen, Linda Van Horn, John Yarnell, Beifan Zhou; for the INTERMAP Research Group
Abstract-Findings from short-term randomized trials indicate that dietary supplements of omega-3 polyunsaturated fatty acids (PFA) lower blood pressure of hypertensive persons, but effect size in nonhypertensive individuals is small and nonsignificant. Data are lacking on food omega-3 PFA and blood pressure in general populations. The International Study of Macro- and Micro-nutrients and Blood Pressure (INTERMAP) is an international cross-sectional epidemiologic
study of 4680 men and women ages 40 to 59 from 17 population-based samples in China, Japan, United Kingdom, and United States. We report associations of food omega-3 PFA intake (total, linolenic acid, long-chain) of individuals with blood pressure. Systolic and diastolic blood pressure were measured 8 times at 4 visits. With several models to control for possible confounders (dietary, other), linear regression analyses showed inverse relationship of total omega-3 PFA from food (percent kilocalories, from four 24-hour dietary recalls) to systolic and diastolic blood pressures. With adjustment for 17 variables, estimated systolic blood pressure/diastolic blood pressure differences with 2 standard deviation higher (0.67% kcal) omega-3 PFA were -0.55/ -0.57 mm Hg (Z-score -1.33, -2.00); for 2238 persons without medical or dietary intervention, -1.01/ -0.98 mm Hg (Z -1.63, -2.25); for 2038 nonhypertensive persons from this sub-cohort, 0.91/ 0.92 mm Hg (Z -1.80, -2.38). For linolenic acid (largely from vegetable foods), blood pressure differences were similar, eg, for the 2238 "nonintervened" individuals, -0.97/- 0.87 mm Hg (Z -1.52, -1.95); blood pressure differences were -0.32/ -0.45 mm Hg for long-chain omega-3 PFA (largely from fish).
In summary, food omega-3 PFA intake related inversely to blood pressure, including in nonhypertensive persons, with small estimated effect size. Food omega-3 PFA may contribute to prevention and control of adverse blood pressure levels.
Discussion
Main findings of this population-based study on food w-3 PFA intake of individuals and their blood pressure are: (1) Consistent independent inverse relations of total w-3 PFA to systolic and diastolic pressure; (2) estimated effect size small, <1.0 mm Hg with 2 SD higher w-3 PFA intake (about 1.9 g/d); (3) estimated effect size larger for nonhypertensive persons and for persons not reporting lifestyle modification (eg, special diet, use of nutritional supplements), diagnosed CVD or diabetes, prescribed medication for major chronic disease; (4) similar inverse relations also of linolenic acid to SBP/DBP; (5) for long-chain w-3 PFA (sum EPA DHA, EPA separately, DHA separately) qualitatively similar weaker inverse relation to DBP.
To the best of our knowledge these INTERMAP data indicating low-order independent inverse relations of food w-3 PFA (total, linolenic acid, long-chain) to BP are the first comprehensive population-based findings on this matter. The
Finnish Kuopio Study of 722 middle-aged men reported a significant independent cross-sectional relation of dietary ALA to SBP and mean arterial pressure, but not to DBP; no data were given on long-chain w-3 PFA.9
These INTERMAP observational data on food w -3 PFA and BP are concordant with results from metaanalyses of randomized trials assessing whether w-3 PFA supplements (mostly fish oil capsules) influence BP; in particular, our data
are similar qualitatively and quantitatively in indicating a low-order favorable BP effect, including in nonhypertensive persons,3-6 and are also compatible with the small nonsignificant differences ( 0.4/ 0.6 mm Hg) reported recently from
the Kanwu Study Group RCT on 162 healthy nonhypertensive adults.18
As to BP influences of ALA per se, Wendland et al8- based on 3 RCTs involving 348 persons-reported small nonsignificant effect sizes (-0.72/ 0.17 mm Hg). Similarly, for long-chain w-3 PFA per se, the 1993 metaanalysis of
RCTs4 estimated effect sizes overall (ie, for all participants, hypertensive and nonhypertensive) for DHA of -1.50/-0.77 mm Hg/gram, and for EPA of -0.93/ -0.53 mm Hg/gram. Given limitations in statistical power, it is consistent with the foregoing that no significant influences of EPA supplements on BP were noted in a recent overview of 4 small RCTs in nonhypertensive people.7 For DHA supplements (4 g/d), that article reported sizable BP effects on 24-hour and day-time ambulatory BP of 56 overweight hypercholesterolemic adults.7 High order collinearity of EPA and DHA intakes from foods limits ability to estimate their
separate influences on BP. Our separate regression analyses on EPA-BP and DHA-BP relations yielded similar low-order associations for each. Because dietary ALA is a metabolic precursor of EPA and DHA,8 it is a reasonable expectation that all these dietary w-3 PFA relate to BP.
Limitations of the INTERMAP findings include: their cross-sectional nature, but they are the only available population-based data on food w-3 PFA (total, linolenic acid, long-chain) and BP, and their results are consistent with those
from RCTs; underestimation of effect size attributable to limited reliability in measurement of nutrients (regression dilution bias); possible confounding of the overall data on food w-3 PFA and BP by special diets, dietary supplements,
and drug treatment for high BP/CVD/diabetes, but the findings prevailed with multivariate control for those and other possible confounders for the 4680 participants. In addition, the data indicating larger influences of w-3 PFA on BP for persons not experiencing such interventions are coherent with the inference that the w-3 PFA-BP relation may be etiologically significant.
Possible mechanisms whereby w-3 PFA may favorably influence BP are, based on animal experimental data: enhanced endothelial vasodilator function,19,20 reduced reactivity of resistant vessel vascular smooth muscle,20,21 increased vascular compliance.22
As noted, if feasible intakes of food w-3 PFA do indeed influence BP favorably for people in the general population, effect size is apparently small, based on INTERMAP and RCT results. This finding, anticipated by INTERMAP, needs to be kept in perspective: First, with multiple nutrients having "small" independent influences, combined effect becomes sizable, ie, improved nutrition is capable of preventing or lowering unfavorable BP levels for most people, as the Dietary Approaches to Stop Hypertension and Optimal Macro-Nutrient Intake Heart feeding trial results indicate. 23-25 Second, long-term effects of habitual eating patterns, from early life into middle-age, may be greater, as data on salt intake and BP indicate.26,27 Third, estimates indicate that lowering of population average SBP by "small" amounts (eg, 2 mm Hg) can result in reduction of mortality rates of 6% for stroke and 4% for coronary heart disease (CHD).26 Fourth, enhanced w-3 PFA intake from foods may contribute to decreased risk of CHD/CVD not only by modestly lowering BP, also by favorably influencing dyslipidemia, by anticoagulant, and antiarrhythmic effects.28-32 Population-wide feasibility of greater w -3 PFA intake from foods, vegetable and marine sources is indicated by findings for INTERMAP Japanese-compared with Chinese, U.K., U.S.A.-participants, ie, linolenic acid and long-chain w-3 PFA both substantially
higher, especially the latter. As to specific food sources, w-3 PFA in 100 g cooked fatty fish (175 kcal) is 2.70g; 100 g canned pink salmon (unsalted) (134 kcal), 1.90g; 20g walnuts (unsalted) (134 kcal), 1.36g; 10g flax seed (45 kcal), 1.83g; 5g canola oil (45 kcal), 0.46g; 5g soy bean oil (45 kcal), 0.34g.
In conclusion, there was a weak inverse relationship to BP of w-3 polyunsaturated fat intake from foods (total, linolenic acid, long-chain) with control for multiple possible confounders. This finding was stronger for nonhypertensive people and persons not experiencing dietary/medical intervention, ie, was stronger after removing sources of possible bias, a result consistent with the inference that the -3 PFA-BP relationship may be etiologically significant, albeit low-order.
Perspectives
Recent research data indicate that multiple improvements in food intake lower BP levels of adults, both prehypertensive and hypertensive. Nutrients possibly accounting for these favorable effects include greater intake of minerals (calcium,
magnesium, phosphorus); vegetable protein; polyunsaturated fatty acids including omega-3; and reduced intake of total fat, saturated fatty acids, cholesterol, sugars-over and above known favorable effects on BP of reduced sodium chloride, increased potassium, and prevention/correction of overweight/
obesity and excess alcohol intake. The findings of the present study indicating a low-order favorable influence of food w-3 fatty acid intake on BP of individuals from general population samples are consistent with metaanalytic data of RCTs on this matter. Thus, these results on a major CHD/CVD risk factor lend modest support to current recommendations for increased ingestion of w-3 fatty acids from marine and vegetable sources.
Unclarity persists concerning efficacy of omega-3 (w -3) polyunsaturated fatty acid (PFA) intake for prevention and control of the cardiovascular diseases (CVD) and their major risk factors. This is particularly the case for populationwide
w-3 PFA from foods. As to w-3 PFA supplements for secondary prevention of CVD, recent reviews/meta-analyses come to diverse conclusions.1,2 Inconsistencies also prevail on influences of supplemental w-3 PFA on blood pressure (BP). Meta-analyses of randomized clinical trials (RCTs) on w-3 PFA supplements reported significant BP reduction overall and in hypertensive participants; significant heterogeneity in systolic BP (SBP) outcomes across trials; only small nonsignificant systolic and diastolic BP (DBP) lowering in
nonhypertensive individuals.3-8 Almost no population-based observational data exist on relation of food -3 PFA of individuals to their BP.9
Possible reasons for the heterogeneous RCT findings on w-3 PFA supplements and BP are: actual effect size is small, particularly in nonhypertensive individuals, hence false-negative findings are probable unless sample sizes are large, and BP is measured repeatedly by high-quality techniques.
In observational studies, data on nutrient intakes and other variables must be extensive and high-quality, enabling characterization of w-3 PFA intake by individuals and control for multiple possible confounders. The population-based International Study of Macro- and Micro-nutrients and Blood Pressure (INTERMAP) Study on nutrients and blood pressure was designed to cope with such problems.10-15 Its basic premises are: multiple nutrients have small independent influences on BP of individuals that in combination yield sizable effects. To detect impact of single nutrients on BP of individuals, it is essential to collect standardized, high-quality data on large samples of diverse populations. Accordingly, INTERMAP surveyed in-depth 4680 men and women ages 40 to 59 from 17 population samples in Japan, Peoplefs Republic of China, United Kingdom, United States, enabling it to address main unanswered questions on -3 PFA intake and BP: (1) Does food w -3 PFA intake of individuals relate
independently to their SBP/DBP? (2) Is this the case throughout the population, including nonhypertensive individuals? (3) Are both linolenic and long-chain w-3 PFA intake independently associated with their SBP/DBP? INTERMAP hypothesized that dietary w-3 PFA intake of individuals is inversely related to their blood pressure.10 Findings on food w -3 PFA and BP are reported here.
Results
Descriptive Statistics
Detailed data are tabulated in the online supplement to this paper (please see Table S1 at http://hyper.ahajournals.org). Average SBP ranged from 117.2 (Japan) to 121.3 mm Hg (PRC); average DBP, from 73.2 (PRC) to 77.3 (UK) mm Hg. Mean BMI and energy intake were lower for Japanese and PRC participants, highest for American. Mean total w-3 PFA intake from foods (g/24 hours and % kcal) was highest in Japan (1.35% kcal), lowest in China (0.55% kcal); linolenic
acid (ALA) was about 78% of all w-3 PFA overall (60% for Japanese, 98% for Chinese). EPA DHA constituted most long-chain w -3 PFA. Main food groups supplying long-chain w-3 PFA were-for all 4680 participants-fish and fish
products (77.3% of all EPA DHA); shellfish and shellfish products (4.9%); red meats, poultry, eggs and their products (19.9%). This pattern prevailed for participants from Japan (90.4% of EPA DHA from fish, shellfish, and their products) and from the US (78.0%), to a lesser extent for those from the UK (56.0%).
Univariate estimates for reliability of the per person values of w-3 PFA intake based on means of 4 24-hour recalls were for all 4680 participants: total w-3 PFA (%kcal)-observed coefficient 45.6% of true coefficient; linolenic acid, 53.3%; EPA, 31.0%; DHA, 31.0%; DPA, 27.6%; EPA DHA, 35.3%; EPA DHA DPA, 35.3%. Subcohort data on reliability were similar to the foregoing. These estimates varied across countries, eg, for total w-3 PFA intake (all 4680 participants): Japan 28.6%; PRC 79.3%; UK 40.8%; US 55.2%. BP reliability estimates were 94.3% for SBP and 93.0% for DBP.
Partial Correlation Data
Food total w-3 PFA (% kcal) was correlated directly with food linoleic acid (partial r= 0.48) and total w-6 PFA (0.48), arachidonic acid (0.23), total monounsaturated fatty acids (MFA; 0.34), oleic acid (0.27), Vitamin E (0.35); inversely with total available carbohydrate ( 0.34) and total sugars (-0.21). ALA was correlated similarly with the foregoing variables; it was not correlated with EPA, DHA, DPA, or their sums. EPA, DHA, DPA were highly intercorrelated (r
values 0.65 to 0.84). Sum EPA+DHA was correlated with total protein (0.30), arachidonic acid (0.36), phosphorus (0.18), vitamin E (0.16), as was sum EPA DHA DPA.
Relation of Food Total Omega-3 PFA to
Blood Pressure
All 4680 Participants
Consistently, dietary total w-3 PFA was inversely related to SBP and DBP (Table 1). With 2 standard deviation higher total w-3 PFA (0.669% kcal about 1.9 g/d), estimated difference in SBP was about -0.4 to -0.6 mm Hg; in DBP, about -0.5 to -0.6 mm Hg (DBP Z-scores -1.71 to - 2.23). For all models on the relation of w-3 PFA to BP, there was no statistically significant interaction with age, gender, or BMI. The inverse w-3 PFA-BP relation was nonsignificantly stronger for US participants, eg, for model 5P, estimated SBP difference of -1.26 mm Hg with 2 SD higher w-3 PFA, DBP -0.80 mm Hg; for PRC participants, DBP difference
-1.73 mm Hg. Analyses with control for urinary Na/creatinine and K/creatinine (instead of 24-hour Na and K excretion) yielded similar findings, eg, with Model 5P DBP difference -0.56 mm Hg (Z -1.96) with w-3 PFA intake 2 SD higher.
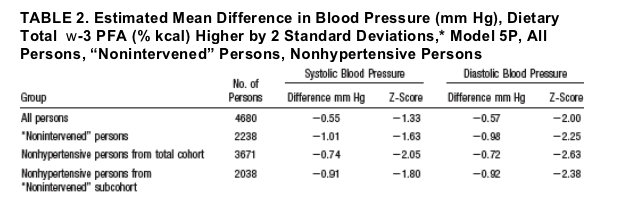
"Nonintervened" Subcohort (n=2238)
Percentage of persons with untreated high BP in this subcohort was: 11.8% for men, 5.7% for women. With 2 SD higher w -3 PFA intake, estimated SBP and DBP differences were consistently greater than for all participants, eg, with model
5P, SBP difference -1.01 and DBP difference -0.98 with 2 SD higher w-3 PFA (Table 2). These differences were nonsignificantly larger for PRC and US participants, SBP -1.28 and -2.22 mm Hg, DBP -2.12 and -2.03 mm Hg.
Nonhypertensive Persons (n=3671 and 2038)
For the 3671 nonhypertensive persons from the total cohort, SBP and DBP differences with 2 SD higher dietary w-3 PFA were greater than for all 4680 participants, eg, SBP difference -0.74 mm Hg and DBP difference -0.72 mm Hg (Model 5P; Table 2). SBP differences were nonsignificantly larger for UK and US participants, -0.89 mm Hg and -1.66 mm Hg; DBP differences, larger for PRC and US participants, -1.22 mm Hg and -1.14 mm Hg. Findings were similar for
nonhypertensive persons from the subcohort of "nonintervened" individuals.
Sensitivity Analyses
Multiple other regression models yielded results qualitatively similar to the foregoing, eg, modifications A-D of Model 5P (Table 3). With Model D, excluding people with high day-to-day variability in SBP, DBP, or nutrient intakes, BP
differences, and Z-scores were greater than for all 4680 persons.
Relation of Linolenic Acid and Long-Chain w-3
PFA From Foods to Blood Pressure
For all 4680 participants and the 3 subcohorts, relations of linolenic acid and long-chain w-3 PFA to BP were similar in models where ALA and sum, EPA+DHA (or sum, EPA+DHA+DPA) were or were not considered together.
ALA Intake From Foods and BP
Consistently, ALA was inversely related to SBP; thus, in Model 5P estimated SBP differences with 2 SD higher intake (0.566% kcal, =about 1.6 g/d) ranged from -0.60 mm Hg (all 4680 participants) to -0.97 mm Hg (2238 "nonintervened"
persons; Table 4). Repeatedly, data for US participants showed nonsignificantly larger estimated SBP differences, eg, with Model 5P differences of -1.50 mm Hg to -1.92 mm Hg across the 4 groups.
The relation of food ALA to DBP was also inverse, with evidence of significant cross-country heterogeneity, eg, Model 5P (Table 4). For the 4 countries separately, estimated DBP differences varied in sign and amount, eg, Model 5P for all 4680 persons: Japan +0.52 mm Hg, PRC -1.52 mm Hg, UK +2.46 mm Hg, US -0.97 mm Hg; similarly for the "nonintervened" subcohort (n 2238): +0.32, -1.90 (Z= -2.35), +2.20, -1.83 (Z = -2.21).
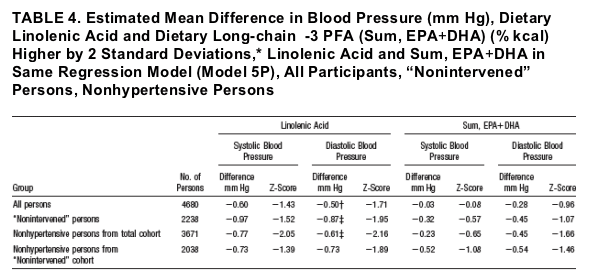
Long-Chain w-3 PFA From Foods and BP
Findings for the relation to BP of Sum EPA DHA DPA and Sum EPA DHA were similar: For the 4 groups, DBP differences with 2 SD higher EPA+DHA (0.318%
kcal, about 0.79 g/d) ranged (model 5 P) from -0.28 mm Hg to - 0.54 mm Hg; SBP differences were generally smaller (Table 4).
Corresponding analyses were done on the relation to BP of EPA and DHA considered separately. Results were qualitatively similar to the foregoing: eg, Model 5P, with 2 SD higher EPA, DBP lower by -0.21 to -0.56 mm Hg; for
DHA, -0.31 to -0.61 mm Hg. Findings were similar from regressions of SBP, DBP on EPA, DHA, EPA+DHA ingested only from fish/shellfish and their products.
In multiple regression models with these 2 highly correlated variables considered together in the same model, the relation of EPA to SBP and DBP varied across cohorts, ie, was nonsignificantly positive for all 4680 participants and the
2238 "nonintervened" persons, nonsignificantly inverse for the 3671 nonhypertensive persons and the subcohort of 2038 nonhypertensive persons. DHA-BP relations also varied in sign across cohorts and all had low-order Z-scores.
Methods
Population Samples, Field Methods (1996 -1999)
INTERMAP included men and women ages 40 to 59 years from population random samples in Japan (4 samples), Peoplefs Republic of China (PRC, 3), United Kingdom (UK, 2), and United States (US, 8).10 Staff were trained and certified for BP measurement by international/national senior colleagues based on a common standardized protocol.10 Each participant attended 4 times, visits 1 and 2 on consecutive days, visits 3 and 4 on consecutive days on average 3 weeks later. For BP measurement, each participant- having emptied his/her bladder-was seated comfortably for 5 minutes, feet flat on the floor, in a quiet room, with no physical activity in the preceding half hour. Korotkoff sounds I and V were criteria for SBP and DBP. BP was measured twice at each visit with a random zero sphygmomanometer; BP at each visit was the average of the 2
readings. Measurements of height and weight, and questionnaire data on daily alcohol consumption over the previous 7 days were obtained at 2 visits. Dietary data were collected at each visit by a trained interviewer with use of the in-depth multi-pass 24-hour recall method.11 All foods and drinks consumed in the previous 24 hours, including dietary supplements, were recorded. Questionnaire data were obtained on demographic and other possible confounders. Quality control was extensive.
Each participant provided 2 24-hour urine collections, start and end timed at the research center (visits 1 to 2 and 3 to 4); measurements included urinary volume, sodium, potassium, creatinine, urea10; 10% of samples were split locally and sent to the Central Laboratory with different identification number to estimate
technical error. Individuals were excluded if they did not attend all 4 visits; diet
data were considered unreliable; energy intake from any 24-hour dietary recall was below 500 or greater than 5000 kcal/d for women, 8000 kcal for men; 2 urine collections were not available; data on other variables were incomplete or indicated protocol violation (total exclusions: 215 people). For each exclusion, a supplementary participant was recruited.
The study received institutional ethics committee approval for each site; all participants gave written consent; all the procedures followed were in accordance with institutional guidelines.
Statistical Methods
Food data of individuals were converted into nutrient intakes (83 nutrients) with use of enhanced country-specific food tables, standardized across countries by the Nutrition Coordinating Center, University of Minnesota.11,12 For nutrients supplying energy, intake was calculated as percent total energy; for others, as intake/1000 kcal; nutrients were calculated also as amounts/24 hours. Food data were used to estimate main food groups supplying w -3 PFA-total, linolenic acid (largely from vegetable sources), long-chain w-3 PFA (largely from fish; eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA], docosapentaenoic acid [DPA]). Urinary values/24 hours were calculated as products of urinary concentrations and timed volume standardized to 24 hours. Measurements/person were averaged, for BP and nutrient variables, across the 4 visits; for the urinary excretions, across the 2 24-hour collections. For descriptive statistics, means, standard deviations, numbers, and percentages were calculated by country and study-wide. Reliability of SBP, DBP, and w-3 PFA intakes from the mean of the 4 visits was estimated from the formula 1/[1 (ratio/4)] 100, where the ratio is intraindividual variance/interindividual variance, estimated separately for 8 gender/country strata and pooled by weighting each stratum-specific estimate by (sample size minus one). This gives a first approximation of reliability, ie, an estimate of the size of an observed coefficient as a per cent of the true coefficient in a univariate regression analysis.16,17
Associations among nutritional variables were explored by partial correlation, adjusted for sample, age, gender; pooled across countries, weighted by sample size. Multiple regression analyses were used to examine relationships of food w-3 PFA (percent kcal) of individuals-total, linolenic acid, long-chain-to their SBP and DBP. These analyses were done for four cohorts: all 4680 participants; 2238 "nonintervened" persons not on a special diet, not consuming nutritional supplements, not with diagnosed CVD/diabetes (DM); not taking medication for high BP, CVD, diabetes; ie, exclusion of people whose data might bias the food -3 PFA-BP relationship; nonhypertensive individuals-SBP <140, DBP <90 mm Hg, not taking antihypertensive medication-from the total cohort (n 3671) and from the "nonintervened" subcohort (n 2038). Adjustment for confounders was done sequentially: for sample, age, gender, weight, height (Model 1); plus reported special diet, dietary supplement intake, moderate/heavy physical activity (hours/d), history of CVD/DM, family history of hypertension (Model 2); plus
24-hour urinary sodium, potassium, (or urinary sodium/creatinine, potassium/creatinine) and 7-day alcohol intake (Model 3); plus dietary cholesterol, saturated fatty acids (SFA), calcium (Model 4); plus dietary fiber or magnesium or phosphorus, separately because of collinearity (Models 5a, 5b, 5P).
Regression models were fitted by country and coefficients pooled across countries, weighted by inverse of variance, to estimate overall association; cross-country heterogeneity was tested; interactions were assessed for age, gender, and body mass index (BMI, weight/height2 [kg/m2]). Regression coefficients were expressed as mm Hg for 2 standard deviation (SD) higher food w -3 PFA, from pooled within-country standard deviations weighted by sample size.
Sensitivity analyses involved: inclusion of energy intake with nutrient densities; use of g/d intakes adjusted for energy; addition to regression models of other nutrients (monounsaturated fatty acids, oleic acid, w-6 PFA, linoleic acid, arachidonic acid, trans fatty acids, vegetable protein, animal protein, estimated total sugars, vitamin E); exclusion from the "nonintervened" subcohort of people taking nonsteroidal antiinflammatory drug (NSAID); exclusion of people with marked intraindividual variability in nutrient intake or SBP, DBP. Analyses were with SAS version 8.02 by Q.C. and I.J.B.
|
|
| |
| |
|
|
|