| |
Lipitor to Reduce LDL in CVD
|
| |
| |
"Outcomes of Using High- or Low-Dose Atorvastatin in Patients 65 Years of Age or Older with Stable Coronary Heart Disease"
Nanette K. Wenger, MD; Sandra J. Lewis, MD; David M. Herrington, MD; Vera Bittner, MD; Francine K. Welty, MD, PhD, for the Treating to New Targets Study Steering Committee and Investigators
From Emory University School of Medicine, Atlanta, Georgia; Northwest Cardiovascular Institute, Portland, Oregon; Wake Forest University School of Medicine, Winston-Salem, North Carolina; University of Alabama at Birmingham, Birmingham, Alabama; and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts.
Annals on Internal Medicine
3 July 2007 | Volume 147 Issue 1 | Pages 1-9
"....Our findings support the recommendations of the recent National Cholesterol Education Program guidelines for use of intensive LDL cholesterol-lowering therapy in high-risk older persons with established CVD (8) and ACC and AHA guidelines to reduce LDL cholesterol levels to much less than 2.6 mmol/L (<100 mg/dL) in any patient with established CHD...
....Editor's Notes: Context, Data on the benefits of intensive lipid-lowering treatment for elderly persons with heart disease are sparse. Contribution: This secondary analysis of a trial examined outcomes of 3809 adults 65 years of age or older with coronary heart disease who were randomly assigned to receive atorvastatin, 80 or 10 mg/d. Patients achieved average low-density lipoprotein cholesterol levels of approximately 1.81 mmol/L (70 mg/dL) and 2.59 mmol/L (100 mg/dL), respectively. Fewer patients who received 80 mg of atorvastatin had major fatal or nonfatal cardiovascular events than did those who received 10 mg of atorvastatin (10.3% vs. 12.6%). Caution: The researchers could not determine whether benefits were due to the higher statin dose, lower achieved cholesterol levels, or both factors.....Subgroup analyses from large, randomized, placebo-controlled clinical trials (2-4) demonstrated that decreasing low-density lipoprotein (LDL) cholesterol levels with statin therapy statistically significantly reduced the risk for CHD in older persons....Recent secondary prevention guidelines from the American Heart Association (AHA) and the American College of Cardiology (ACC) state that it is reasonable to reduce LDL cholesterol levels to less than 1.8 mmol/L (<70 mg/dL) in any patient with established CHD...In the TNT (Treating to New Targets) study, intensive lipid-lowering treatment with 80 mg of atorvastatin in patients with stable CHD provided clinically significant benefit beyond treatment with 10 mg of atorvastatin (11). Our prespecified secondary analysis reports data from the TNT study on the efficacy and safety of high-dose atorvastatin treatment in patients 65 years of age or older....During the open-label period, LDL cholesterol levels among patients 65 years of age or older decreased from 4.2 mmol/L (163 mg/dL) to 2.5 mmol/L (96 mg/dL). At week 12, mean LDL cholesterol levels were 1.9 mmol/L (72 mg/dL) among those who received 80 mg of atorvastatin and 2.5 mmol/L (97 mg/dL) among those who received 10 mg. Levels of LDL cholesterol in both groups remained stable for the duration of the study.....Among patients 65 years of age or older, a primary event occurred in 199 patients (10.3%) who received 80 mg of atorvastatin and 235 patients (12.6%) who received 10 mg. This is a 2.3% absolute reduction in the rate of major cardiovascular events and a 19% relative reduction in risk in favor of the high-dose group (hazard ratio, 0.81 [95% CI, 0.67 to 0.98]; P = 0.032).... The risk for any cardiovascular event (P < 0.001), a major coronary event (P = 0.128), any coronary event (P < 0.001), a cerebrovascular event (P = 0.010), and hospitalization for congestive heart failure (P = 0.008) was lower in the 80-mg group than in the 10-mg group. The 2 groups did not statistically significantly differ for all-cause mortality and for rates of death due to cardiovascular and noncardiovascular causes.....The rate of death due to cardiovascular causes was lower in the 80-mg group than in the 10-mg group (78 patients [4.0%] vs. 83 patients [4.4%]; hazard ratio, 0.91 [CI, 0.67 to 1.24]; P = 0.55). However, more patients in the 80-mg group than the 10-mg group died of noncardiovascular causes (98 patients [5.1%] vs. 76 patients [4.1%]; hazard ratio, 1.26 [CI, 0.93 to 1.70]; P = 0.129). These hazard ratios are consistent with those in the overall population (0.81 [CI, 0.64 to 1.03] for cardiovascular death [P = 0.085] and 1.25 [CI, 0.99 to 1.57] for noncardiovascular death [P = 0.065])..... The rate of treatment-related adverse events among patients age 65 years or older was 8.3% for the 80-mg group and 5.2% for the 10-mg group. In the cohort with older patients, 4.4% of those who received 80 mg and 2.2% of those who received 10 mg withdrew from the study because of adverse events that the investigator considered to be associated with the treatment. The rates of persistent (occurring twice within 4 to 10 days) elevations in alanine aminotransferase or aspartate aminotransferase levels to greater than 3 times the upper limit of normal were 1.3% (n = 24) in the 80-mg group and 0.1% (n = 1) in the 10-mg group. No documented cases of persistent elevation in creatine kinase levels to greater than 10 times the upper limit of normal occurred.....Statins must be used with increased vigilance in older patients. For patients 65 years of age or older, the small differences between treatment groups in adverse event profiles were similar to that observed in the overall study sample (11). The improved clinical outcome in these patients was not associated with persistent elevations in creatine kinase levels. Also, occurrence of liver function abnormalities was low and was consistent at both doses with levels reported in other large-scale trials of atorvastatin....Although older patients treated with 80 mg of atorvastatin had a slightly lower relative risk reduction than those who received 10 mg compared with younger persons, the absolute clinical benefit (2.3%) was the same in both age groups. Kaplan-Meier curves for the 2 age cohorts show that patients 65 years of age or older who received 80 mg had a reduced risk for major cardiovascular events that approximately equaled that observed for patients younger than 65 years who received 10 mg."
ABSTRACT
Background: Increased life expectancy is associated with an increase in the burden of chronic cardiovascular disease.
Objective: To assess the efficacy and safety of high-dose atorvastatin in patients 65 years of age or older.
Design: A prespecified secondary analysis of the Treating to New Targets study, a randomized, double-blind clinical trial.
Setting: 256 sites in 14 countries participating in the Treating to New Targets study.
Participants: 10 001 patients (3809 patients ≥65 years of age) with coronary heart disease (CHD) and low-density lipoprotein cholesterol levels less than 3.4 mmol/L (<130 mg/dL).
Intervention: Patients were randomly assigned to receive atorvastatin, 10 or 80 mg/d.
Measurements: The primary end point was the occurrence of a first major cardiovascular event (death from CHD, nonfatal non-procedure-related myocardial infarction, resuscitated cardiac arrest, or fatal or nonfatal stroke).
Results: In patients 65 years of age or older, absolute risk was reduced by 2.3% and relative risk by 19% for major cardiovascular events in favor of the high-dose atorvastatin group (hazard ratio, 0.81 [95% CI, 0.67 to 0.98]; P = 0.032). Among the components of the composite outcome, the mortality rates from CHD, nonfatal non-procedure-related myocardial infarction, and fatal or nonfatal stroke (ischemic, embolic, hemorrhagic, or unknown origin) were all lower in older patients who received high-dose atorvastatin, although the difference was not statistically significant for each individual component. The improved clinical outcome in patients 65 years of age or older was not associated with persistent elevations in creatine kinase levels.
Limitation: Because the study was a secondary analysis, the findings should be interpreted within the context of the main study results.
Conclusions: The analysis suggests that additional clinical benefit can be achieved by treating older patients with CHD more aggressively to reduce low-density lipoprotein cholesterol levels to less than 2.6 mmol/L (<100 mg/dL). The findings support the use of intensive low-density lipoprotein cholesterol-lowering therapy in high-risk older persons with established cardiovascular disease.
The age profile of the population in most industrialized countries is changing as life expectancy increases. Because cardiovascular risk increases steadily with age, this demographic transition is associated with an increase in the burden of chronic cardiovascular disease (CVD), including coronary heart disease (CHD) and stroke (1).
Subgroup analyses from large, randomized, placebo-controlled clinical trials (2-4) demonstrated that decreasing low-density lipoprotein (LDL) cholesterol levels with statin therapy statistically significantly reduced the risk for CHD in older persons. On the basis of these early trial data, the Third Report of the National Cholesterol Education Program Adult Treatment Panel (5) recommended that persons older than 65 years of age should not be denied the benefits of lipid-lowering therapy. Since publication of the panel's report, results of the Heart Protection Study (6) and PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) (7) further support the efficacy and safety of statin treatment in older persons. The outcomes of these 2 studies, along with previous evidence, led the National Cholesterol Education Program to conclude that these data provide a strong justification for intensive LDL cholesterol-lowering therapy in high-risk older persons with established CVD (8).
Recent secondary prevention guidelines from the American Heart Association (AHA) and the American College of Cardiology (ACC) state that it is reasonable to reduce LDL cholesterol levels to less than 1.8 mmol/L (<70 mg/dL) in any patient with established CHD (9). In the ACC and AHA guidelines (10), the writing group acknowledged that elderly patients were underrepresented in many clinical trials and urged physicians and patients to participate in trials that will provide additional evidence for therapeutic strategies in elderly patients.
In the TNT (Treating to New Targets) study, intensive lipid-lowering treatment with 80 mg of atorvastatin in patients with stable CHD provided clinically significant benefit beyond treatment with 10 mg of atorvastatin (11). Our prespecified secondary analysis reports data from the TNT study on the efficacy and safety of high-dose atorvastatin treatment in patients 65 years of age or older.
Methods
Study Design and Patients
Details of the TNT study design and outcome measures are published elsewhere (11, 12). After a washout phase, men and women 35 to 75 years of age with established CHD, LDL cholesterol levels between 3.4 and 6.5 mmol/L (130 and 250 mg/dL), and triglyceride levels less than 6.8 mmol/L (<600 mg/dL) were eligible to enter an 8-week, open-label, run-in period with atorvastatin, 10 mg/d. At the end of the run-in phase, 10 001 patients with LDL cholesterol levels less than 3.4 mmol/L (<130 mg/dL) were randomly assigned to receive double-blind therapy with atorvastatin, 10 or 80 mg/d. The time of randomization was used as the baseline, and patients were followed for a median of 4.9 years. The primary study outcome was the time to the first occurrence of a major cardiovascular event, defined as death due to CHD, nonfatal non-procedure-related myocardial infarction, resuscitated cardiac arrest, and fatal or nonfatal stroke. The prespecified secondary outcomes were a major coronary event, a cerebrovascular event, peripheral arterial disease, hospitalization with a primary diagnosis of congestive heart failure, death from any cause, any cardiovascular event, and any coronary event. An independent end point committee that was blinded to treatment assignment adjudicated all primary and secondary outcomes.
Statistical Analysis
We tested the statistical significance of treatment effect on end points by using the log-rank test. We calculated hazard ratios with 95% CIs from a Cox regression model that we present where appropriate. We performed homogeneity tests for treatment interaction with age by using a Cox proportional hazards model to determine whether the treatment effects observed in patients 65 years of age or older differed from those in patients younger than 65 years.
Role of the Funding Source
The TNT study was funded by Pfizer. The steering committee developed the protocol in collaboration with the funding source and was responsible for the final version. ICON Clinical Research, North Wales, Pennsylvania, managed all data. ICON and Pfizer provided site monitoring throughout the study. The data were analyzed by the funding source according to the statistical analysis plan approved by the steering committee. The steering committee had unrestricted, request-based access to the study data, which were retained by the funding source, and developed the article independently without constraints from the sponsor.
Results
Sample
Of 10 001 patients randomly assigned in the overall TNT study cohort, 3809 (38%) were 65 years of age or older (1872 received 10 mg of atorvastatin and 1937 received 80 mg). Baseline characteristics and LDL, high-density lipoprotein, and total cholesterol and triglyceride levels were similar between the 2 treatment groups (Table 1). The mean age of the older cohort was 69.9 years. In this group, 2033 patients were 65 to 69 years of age (1000 received 10 mg of atorvastatin and 1033 received 80 mg) and 1776 patients were 70 years of age or older (872 received 10 mg and 904 received 80 mg). The demographic and cardiovascular profiles of patients age 70 years or older were similar to those of the total elderly cohort, including lipid values and previous CVD at baseline.
Lipid Values
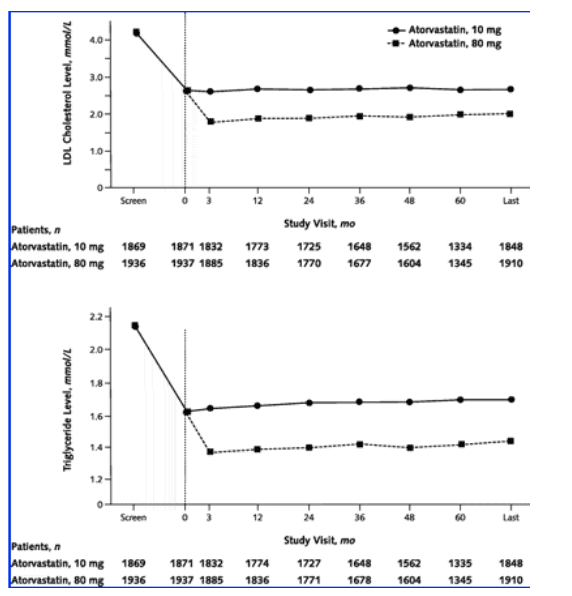
During the open-label period, LDL cholesterol levels among patients 65 years of age or older decreased from 4.2 mmol/L (163 mg/dL) to 2.5 mmol/L (96 mg/dL). At week 12, mean LDL cholesterol levels were 1.9 mmol/L (72 mg/dL) among those who received 80 mg of atorvastatin and 2.5 mmol/L (97 mg/dL) among those who received 10 mg. Levels of LDL cholesterol in both groups remained stable for the duration of the study.
Total cholesterol and triglyceride levels decreased from baseline to week 12 in patients who received 80 mg of atorvastatin and were maintained at this reduced level for the duration of the study. High-density lipoprotein cholesterol levels changed little from baseline levels: At study end, levels had increased by 0.3% for patients who received 10 mg and 0.17% for patients who received 80 mg. Figure 1 shows postrandomization LDL cholesterol and triglyceride levels among patients 65 years of age or older.
Figure 1. Mean low-density lipoprotein (LDL) cholesterol levels (top) and mean triglyceride levels (bottom) among patients 65 years of age or older.
To convert LDL cholesterol values to mg/dL, divide by 0.02586. To convert triglyceride values to mg/dL, divide by 0.01129.
Efficacy Outcomes among Older Patients
Among patients 65 years of age or older, a primary event occurred in 199 patients (10.3%) who received 80 mg of atorvastatin and 235 patients (12.6%) who received 10 mg. This is a 2.3% absolute reduction in the rate of major cardiovascular events and a 19% relative reduction in risk in favor of the high-dose group (hazard ratio, 0.81 [95% CI, 0.67 to 0.98]; P = 0.032) (Table 2). After adjustment for well-established risk factors (sex, race, smoking status, history of diabetes, and history of hypertension), the risk reductions associated with 80 mg were similar to the unadjusted results (hazard ratio, 0.81 [CI, 0.67 to 0.98]; P = 0.032). The number needed to treat for benefit for 80 mg versus 10 mg was 35. This value is the number of patients who need to be treated to prevent 1 cardiovascular event over 4.9 years.
Table 2 shows the incidence of each component of the primary composite outcome among older patients. Rates of death due to CHD, nonfatal non-procedure-related myocardial infarction, and fatal and nonfatal stroke (ischemic, embolic, hemorrhagic, or unknown origin) were lower in the 80-mg group than in the 10-mg group. For each individual component, however, the difference was not statistically significant. Eight patients (0.4%) who received 80 mg and 15 patients (0.8%) who received 10 mg had hemorrhagic stroke, which caused 3 deaths in each group. The risk for any cardiovascular event (P < 0.001), a major coronary event (P = 0.128), any coronary event (P < 0.001), a cerebrovascular event (P = 0.010), and hospitalization for congestive heart failure (P = 0.008) was lower in the 80-mg group than in the 10-mg group. The 2 groups did not statistically significantly differ for all-cause mortality and for rates of death due to cardiovascular and noncardiovascular causes.
The rate of death due to cardiovascular causes was lower in the 80-mg group than in the 10-mg group (78 patients [4.0%] vs. 83 patients [4.4%]; hazard ratio, 0.91 [CI, 0.67 to 1.24]; P = 0.55). However, more patients in the 80-mg group than the 10-mg group died of noncardiovascular causes (98 patients [5.1%] vs. 76 patients [4.1%]; hazard ratio, 1.26 [CI, 0.93 to 1.70]; P = 0.129). These hazard ratios are consistent with those in the overall population (0.81 [CI, 0.64 to 1.03] for cardiovascular death [P = 0.085] and 1.25 [CI, 0.99 to 1.57] for noncardiovascular death [P = 0.065]).
Cancer accounted for more than one half of deaths due to noncardiovascular causes in older patients who received 80 mg (55 patients [2.8%]) and 10 mg (40 patients [2.1%]). No specific body system or type of cancer contributed disproportionately to the small difference in deaths due to cancer between the treatment groups. Rates of death from nontraumatic causes other than cancer were similar between the 80-mg group and 10-mg group (39 patients [2.0%] vs. 31 patients [1.7%], respectively). Deaths from suicide, homicide, and other traumatic causes were infrequent (4 patients [0.2%] vs. 5 patients [0.3%], respectively).
Safety Outcomes among Older Patients
The rate of treatment-related adverse events among patients age 65 years or older was 8.3% for the 80-mg group and 5.2% for the 10-mg group. In the cohort with older patients, 4.4% of those who received 80 mg and 2.2% of those who received 10 mg withdrew from the study because of adverse events that the investigator considered to be associated with the treatment. The rates of persistent (occurring twice within 4 to 10 days) elevations in alanine aminotransferase or aspartate aminotransferase levels to greater than 3 times the upper limit of normal were 1.3% (n = 24) in the 80-mg group and 0.1% (n = 1) in the 10-mg group. No documented cases of persistent elevation in creatine kinase levels to greater than 10 times the upper limit of normal occurred.
Efficacy and Safety among Younger Patients versus Older Patients
Patients 65 years of age or older were more likely than patients younger than 65 years of age to be female and to be white. Hypertension and diabetes mellitus were more prevalent and current cigarette smoking was less prevalent among older patients than younger patients. Older patients more often than younger patients had a history of angina, coronary artery bypass graft surgery, cerebrovascular disease, peripheral arterial disease, and congestive heart failure but were less likely to have had a previous myocardial infarction or a previous coronary angioplasty.
The relative reduction in the rate of major cardiovascular events was slightly higher (24%) in the younger group than in the older group, although the absolute risk reduction was the same (2.3%; hazard ratio, 0.76 [CI, 0.64 to 0.90]; P = 0.001) (Figure 2, top). For the younger patients, the number needed to treat for benefit for 80 mg versus 10 mg was 26.
The relative reduction in the risk for stroke was also higher (30%) in younger patients than in older patients (hazard ratio, 0.70 [CI, 0.49 to 1.00]; P = 0.048), although the absolute risk reduction (0.7%) was similar (Figure 2, bottom). Among the younger patients, 8 (0.3%) who received 80 mg of atorvastatin and 3 (0.1%) who received 10 mg had hemorrhagic stroke.
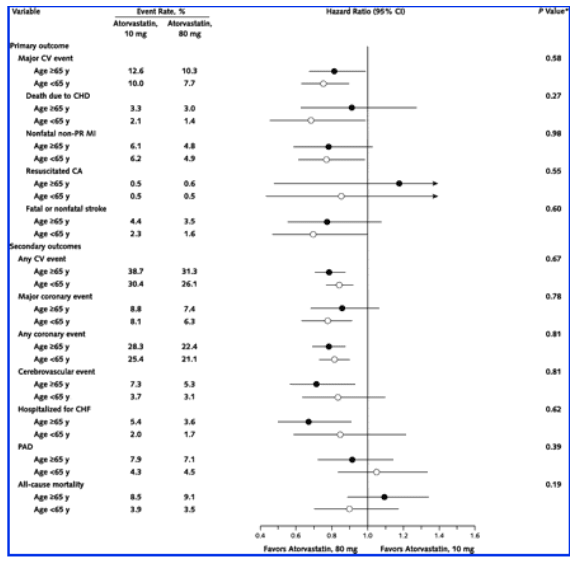
No statistically significant heterogeneity of treatment effect for age was evident for the primary outcome (or its individual components) or secondary outcomes (Figure 3). Hazard ratios for younger and older patients were similar to those for the overall study sample. For older and younger patients, there were small differences between the 80-mg and 10-mg groups in adverse events and withdrawals related to treatment (Table 3). Rates of liver function abnormalities were consistent for both doses and were similar for younger and older patients (Table 3). No patient younger than 65 years experienced persistent elevations in creatine kinase levels.
Figure 3. Hazard ratios and heterogeneity tests for primary and secondary outcomes among patients 65 years of age or older (solid circles) and those younger than 65 years of age (open circles).
Discussion
Effect of Intensive Lipid-Lowering Treatment in Patients 65 Years of Age or Older
For patients 65 years of age or older with stable CHD, intensive lipid-lowering treatment with 80 mg of atorvastatin statistically significantly reduced the rate of major cardiovascular events compared with 10 mg of atorvastatin. Our analysis, which involves 2 active therapies, extends the findings of previous placebo-controlled studies (6, 7) and, consistent with current ACC and AHA secondary prevention recommendations (9), suggests that additional clinical benefit can be achieved by aggressively treating older patients to reduce LDL cholesterol levels to less than 2.6 mmol/L (<100 mg/dL).
Prevention of stroke among older patients is of great importance because of the potential for severe long-term physical and mental disability and the large associated economic cost (14). Rates of stroke in the TNT study are indicative of the increased burden of stroke among elderly persons. Among patients 65 years of age or older, the incidence of fatal stroke, nonfatal stroke, or both was approximately twice that observed among younger patients (in contrast to the rates of coronary events, which were similar between the 2 age cohorts). In our analysis, the rate of fatal and nonfatal stroke in patients 65 years of age or older was lower in the 80-mg group than in the 10-mg group, although the difference was not statistically significant. Other studies (3, 6, 15) have demonstrated the benefit of statin therapy (compared with placebo) in reducing the risk for stroke in older patients. In contrast to the TNT study, however, statistical significance in these trials was reached among older patients. In PROSPER (7), no reduction in stroke was observed among patients older than 70 years of age who received statin therapy compared with those who received placebo; this finding may have been due to a shorter duration of follow-up.
All cardiovascular and coronary events were statistically significantly reduced in older patients who received 80 mg of atorvastatin compared with those who received 10 mg. Among older patients, intensive lipid-lowering treatment was also associated with reductions in the risk for major coronary events, cerebrovascular events, and hospitalization for congestive heart failure (which reached statistical significance for cerebrovascular events and congestive heart failure) for those who received 80 mg compared with those who received 10 mg. For older patients, and consistent with the overall population, the 2 treatment groups did not statistically significantly differ in death from any cause. In the TNT study, the overall mortality rate was low, and the rate of death due to noncardiovascular causes exceeded the rate of death due to cardiovascular causes among patients who received intensive lipid-lowering therapy. As a result, we cannot confidently rule out a small increase or decrease in overall death in the total cohort or in the 2 age-defined subgroups that we examined.
Statins must be used with increased vigilance in older patients. For patients 65 years of age or older, the small differences between treatment groups in adverse event profiles were similar to that observed in the overall study sample (11). The improved clinical outcome in these patients was not associated with persistent elevations in creatine kinase levels. Also, occurrence of liver function abnormalities was low and was consistent at both doses with levels reported in other large-scale trials of atorvastatin (16-18).
Comparison of Effect
Although older patients treated with 80 mg of atorvastatin had a slightly lower relative risk reduction than those who received 10 mg compared with younger persons, the absolute clinical benefit (2.3%) was the same in both age groups. Kaplan-Meier curves for the 2 age cohorts show that patients 65 years of age or older who received 80 mg had a reduced risk for major cardiovascular events that approximately equaled that observed for patients younger than 65 years who received 10 mg (Figure 3).
The numbers needed to treat for benefit based on these results were low for both older and younger patients (35 for patients ≥ 65 years vs. 26 for patients < 65 years). The values represent the number of patients who must be treated with intensive lipid-lowering therapy over 4.9 years to prevent 1 additional cardiovascular event greater than that achievable by using a moderate drug regimen.
Secondary analyses and meta-analyses of large randomized, placebo-controlled trials have also demonstrated that the cardiovascular benefits of statin therapy observed in a sample of older patients with CHD are similar to those in younger patients. In the Heart Protection Study (6), the absolute risk reduction in major vascular events compared with placebo among patients 65 years of age or older who received simvastatin (5.6%) was slightly higher than that in patients younger than 65 years (5.2%). Investigators reported similar results in the Cholesterol Treatment Trialists' collaboration, a prospective meta-analysis of more than 90 000 patients in 14 randomized trials of statins (19). Although most studies in the Cholesterol Treatment Trialists' analysis are of samples different from that in the TNT study (and involve relatively low statin doses), the absolute risk reduction was the same in patients older than 65 years and those 65 years or younger. The PROSPER (7), the only major trial to our knowledge that was conducted exclusively in older individuals (age 70 to 82 years), also showed a significant reduction in major cardiovascular events among patients who received statin therapy compared with those who received placebo, but it showed no reduction in risk for stroke. Other analyses of the effects of statin treatment in patients with CHD have demonstrated higher absolute reductions in the risk for major coronary events among older patients compared with younger patients (2-4).
The small increase in the rates of treatment-related adverse events and withdrawals among older patients who received 80 mg of atorvastatin compared with that of younger patients is consistent with that reported in previous statin trials (2).
Although our findings add to previous data about the benefit of decreasing LDL cholesterol levels in elderly patients, our analysis has limitations. Inclusion of more older patients would have strengthened our ability to detect subtle treatment effects with confidence. Nevertheless, with more than 3800 older patients and 400 primary outcomes, we detected a roughly 20% treatment effect at the 0.03 level of statistical significance. The ability to detect treatment effects of similar size for some less frequent secondary outcomes was limited.
In addition, we could not determine whether the clinical benefit observed among older patients was related to the higher statin dose, lower resultant LDL cholesterol levels, or both factors. Thus, we recommend that physicians choosing dose titration for patients 65 years of age or older be guided by clinical judgment and existing treatment guidelines. The safety data we present should also encourage physicians to follow titration guidelines for this age group.
In conclusion, patients 65 years of age or older treated with intensive lipid-lowering therapy with 80 mg of atorvastatin experienced additional benefit beyond that achieved with 10 mg of atorvastatin in preventing potentially disabling cardiovascular events. Older patients who received 80 mg had reduced risk for major cardiovascular events that was approximately the same as that for patients younger than 65 years treated with 10 mg. Although treatment with 80 mg conferred a smaller relative risk reduction for older patients than for younger patients, the absolute risk reduction remained as high. Our findings support the recommendations of the recent National Cholesterol Education Program guidelines for use of intensive LDL cholesterol-lowering therapy in high-risk older persons with established CVD (8) and ACC and AHA guidelines to reduce LDL cholesterol levels to much less than 2.6 mmol/L (<100 mg/dL) in any patient with established CHD (9).
|
|
| |
| |
|
|
|