| |
The Effect of Low-Dose Ritonavir Monotherapy on Fasting Serum Lipid Concentrations
|
| |
| |
S.D. Shafran, L.D. Mashinter and S.E. Roberts, Division of Infectious Diseases, Department of Medicine, University of Alberta, Edmonton, Alberta, Canada
HIV Med. 2005;6(6):421-425.
Abstract and Introduction
Abstract
Objectives: Ritonavir (RTV) at doses of 400 mg twice a day (bid) or higher adversely affects serum lipids. However, the effect of RTV 100 mg bid on serum lipids is unknown. We conducted a study to evaluate the effect of RTV 100 mg bid on fasting serum lipid profiles in HIV-negative healthy volunteers.
Methods: Ritonavir 100 mg bid was administered for 14 days to 20 healthy HIV-seronegative adults with normal serum lipids. After a 7-day washout, lopinavir/ritonavir (LPV/RTV) 400/100 mg bid was administered for 14 days. Fasting serum lipid parameters were measured twice at baseline, after 14 days of RTV, and after 14 days of LPV/RTV, and comparisons were made at each time-point for levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, the total/HDL cholesterol ratio and triglycerides.
Results: After 14 days of RTV 100 mg bid, total cholesterol level increased by 10.2% (P<0.001), LDL cholesterol level increased by 16.2% (P<0.001), triglyceride levels increased by 26.5% (P<0.001), HDL cholesterol level decreased by 5.4% (P<0.01) and the total/HDL cholesterol ratio increased by 17.3% (P<0.001). The addition of LPV 400 mg bid to RTV 100 mg bid resulted in no significant further changes in LDL cholesterol or triglyceride level or total/HDL cholesterol ratio, but there were significant increases in both total cholesterol (8.0% increase; P=0.007) and HDL cholesterol levels (6.7% increase; P=0.008).
Conclusions: Ritonavir dosed at 100 mg bid significantly increased the concentration of total cholesterol, LDL cholesterol, total/HDL cholesterol ratio and triglycerides and reduced HDL cholesterol concentration. The addition of LPV 400 mg bid to RTV 100 mg bid further increased both total and HDL cholesterol levels without affecting the total/HDL ratio.
Introduction
Treatment of HIV/AIDS changed dramatically in the mid-1990s with the introduction of highly active antiretroviral therapy (HAART), which initially consisted of one or more HIV protease inhibitors (PIs) together with two nucleoside reverse transcriptase inhibitors (NRTIs). PI-based HAART resulted in dramatic reductions in mortality,[1] hospitalization,[2] and the incidence of most opportunistic infections.[1,3] However, PI therapy has been associated with a number of adverse effects, including hyperlipidaemia.[4] Preliminary data suggest that PI-induced hyperlipidaemia is associated with an increased risk of atherosclerotic heart and cerebrovascular disease,[5] as with genetic/dietary hyperlipidaemia. When the PI ritonavir (RTV) was introduced, it was used as the sole PI therapy at a dosage of 600 mg twice daily. The initial studies of RTV monotherapy demonstrated that RTV caused significant increases in serum cholesterol, and marked increases in serum triglycerides.[6,7] In a study of 11 HIV-negative volunteers who received 2 weeks of RTV 500 mg twice daily, fasting cholesterol level increased by 23.6% and fasting triglyceride levels increased by 146%.[8] Cohort studies have reported higher rates of hyperlipidaemia, principally hypertriglyceridaemia, with RTV than with other PIs.[9-13]
In recent years, the use of RTV at 'full' doses of 600 mg twice daily has nearly disappeared from clinical practice. However, use of lower doses of RTV, 100-400 mg daily, to 'boost' serum concentrations of the PIs amprenavir, atazanavir, fosamprenavir, indinavir, lopinavir (LPV) and saquinavir by inhibiting their metabolism by cytochrome P450 3A4 is increasingly common. Indeed, LPV is only available in fixed dosage combination with RTV, because of the unfavourable pharmacokinetic profile of LPV when not boosted by RTV. The investigational PIs tipranavir and TMC 114 are currently under study exclusively with RTV for boosting, also because of unfavourable pharmacokinetic profiles without RTV boosting.
In comparison with nelfinavir (NFV)-treated subjects, LPV/RTV-treated subjects developed significantly higher rates of grade 3 and 4 hypertriglyceridaemia, as well as higher rates of grade 3 and 4 hypercholesterolaemia,[14] although the latter difference was not statistically significant. The precise magnitude of the lipid perturbations attributable to LPV/RTV or NFV in that clinical trial are not clear, because serum lipid concentrations were measured on blood samples collected without regard to fasting, and because patients in this study received stavudine, which also causes hyperlipidaemia.[15]
To more accurately characterize the hyperlipidaemic effects of LPV/RTV, and to determine the relative contributions of RTV and LPV to the hyperlipidaemia, we conducted a study in HIV-seronegative healthy adult volunteers who received only RTV or LPV/RTV without other drugs, and measured serum lipid concentrations only in the fasting state.
Methods
This study was approved by the Health Research Ethics Committee of the University of Alberta.
Subjects
Study subjects were 10 male and 10 female volunteers aged 18 years or older, who tested HIV-seronegative at enrolment and were taking no medications with the exception of vitamins or replacement doses of L-thyroxine. Study subjects were required to have fasting serum cholesterol concentration <6.2 mmol/L, fasting serum triglyceride concentration <2.0 mmol/L and fasting glucose concentration <7.0 mmol/L. The baseline serum alanine aminotransferase and alkaline phosphatase concentrations had to be less than 1.5 times the upper limit of normal, and female participants were required to have a negative urine pregnancy test prior to administration of study medication, as well as to avoid pregnancy during the study.
Study Procedures
After providing signed informed consent, study subjects underwent a physical examination and had a history taken. The physical examination had to be within normal limits for study participation. Two blood samples were collected after an overnight fast (minimum 12 h) and a serum lipid profile was determined, which consisted of concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol and triglycerides, and total/HDL cholesterol ratio. Total cholesterol levels, HDL cholesterol and triglyceride levels were measured directly enzymatically. LDL cholesterol concentration was calculated using the Friedewald formula. The Friedewald formula cannot be used when triglyceride levels exceed 10.3 mmol/L, but that did not occur in this study. The total/HDL cholesterol ratio was calculated directly.
Within 14 days of the first baseline blood sample, study subjects took RTV 100 mg twice daily orally for 14 days. The next morning, blood was collected in the fasting state for serum lipid profile to assess the effect of RTV alone. Then, the subjects underwent a 7-day washout during which they received no PI. Following the 7-day washout, the subjects received LPV/RTV 400/100 mg twice daily orally for 14 days, given as commercial Kaletra capsules (Abbott Laboratories, Montreal, Canada). On the morning following the last dose of LPV/RTV, a blood sample was collected in the fasting state for serum lipid profile to assess the effect of LPV/RTV.
Statistical Analysis
The baseline serum lipid parameters used for analysis were the mean values of the two baseline fasting samples. The effects of RTV and LPV/RTV on the five components of the fasting serum lipid profile were examined by comparing the mean baseline values with those obtained on day 15 (after 14 days of RTV 100 mg twice daily) and those obtained on day 35 (after 14 days of LPV/RTV 400/100 mg twice daily), as well as by comparing day 35 and 15 values. Two-sided Student's t-tests were used for all comparisons, and the level of significance was taken as 0.05. Comparisons were also undertaken between men and women.
Results
Ten men and 10 women were enrolled with a mean age of 31.5 years (range 18-54 years). The mean baseline lipid parameters were as follows: total cholesterol concentration 4.30 mmol/L (range 3.08-5.47 mmol/L), HDL cholesterol concentration 1.39 mmol/L (range 0.94-1.80 mmol/L), LDL cholesterol concentration 2.51 mmol/L (range 1.53-3.32 mmol/L), triglyceride concentrations 0.87 mmol/L (range 0.38-1.52 mmol/L) and total/HDL cholesterol ratio 3.16 (range 2.25-5.00). One study subject initially enrolled but not included in the demographic figures above discontinued RTV therapy after 4 doses of LPV/RTV because of symptoms of numbness of the tongue and back of the throat and slurred speech for 6 h, followed by 2 days of nausea, fatigue and myalgias. These adverse effects resolved completely and this subject was replaced. No serious adverse effects were observed during the study. Minor adverse effects, principally gastrointestinal, were experienced by 60% of subjects while receiving RTV and 90% of subjects while receiving LPV/RTV.
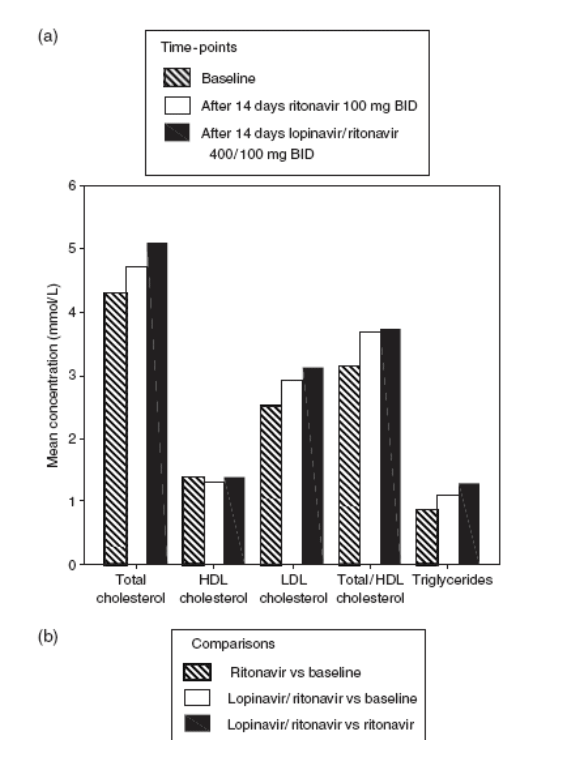
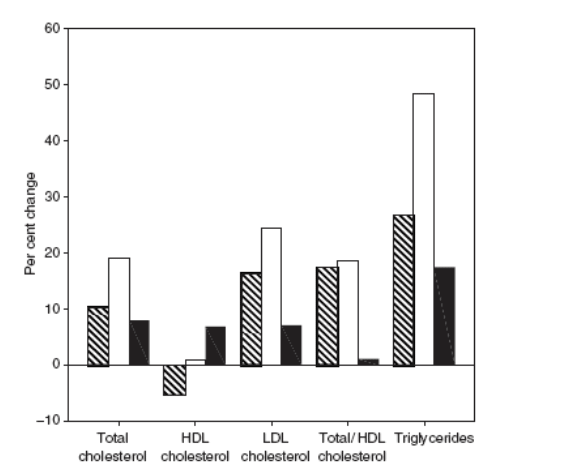
The effects of RTV 100 mg twice daily and of LPV/RTV 400/100 mg twice daily on serum lipid parameters are listed in Fig. 1. Ritonavir 100 mg twice daily resulted in statistically significant increases in the levels of total cholesterol (P< 0.001), LDL cholesterol (P<0.001) and triglycerides (P<0.001), and total/HDL cholesterol ratio (P<0.001), as well as a statistically significant reduction in HDL cholesterol concentration (P=0.01). The magnitude of the change from baseline was greatest with triglycerides (26.5%). The addition of LPV 400 mg twice daily to RTV 100 mg twice daily resulted in a statistically significant further increase in both total cholesterol (P=0.007) and HDL cholesterol (P=0.008) levels without further increasing the total/HDL cholesterol ratio. There were also further rises in LDL cholesterol and triglyceride levels, although these were not statistically significant. In comparison with baseline values, LPV/RTV therapy was associated with a statistically significant increase in total cholesterol (P<0.001), LDL cholesterol (P<0.001) and triglyceride (P=0.015) levels and total/HDL cholesterol ratio (P<0.001), but no significant change in HDL cholesterol concentration. Separate analyses of men and women found no appreciable differences (data not shown).
Figure 1. Fasting lipid parameters pre- and post-drug exposure, showing (a) the mean results in mmol/L, and (b) the per cent change. bid, twice a day; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Discussion
In this study of HIV-seronegative volunteers, we confirmed the finding obtained in clinical trials and clinical practice with HIV-infected individuals that LPV/RTV is associated with hyperlipidaemia, especially hypertriglyceridaemia.[14,16] We also demonstrated that RTV 100 mg twice daily, a dose insufficient for an antiretroviral effect but adequate for inhibition of the cytochrome P450 3A4 enzyme, and a dose commonly used clinically to boost other PIs, is associated with statistically significant adverse effects on the serum lipid profile, characterized by an increase in the concentrations of total cholesterol, LDL cholesterol and triglycerides, and total/HDL cholesterol ratio, as well as a decrease in HDL cholesterol concentration. The addition of LPV 400 mg twice daily to RTV 100 mg twice daily was associated with a normalization of HDL cholesterol level and a further significant increase in total serum cholesterol level.
This study is unique, in that it is the first to examine the effect of RTV 100 mg twice daily given alone, without other drugs which may have a propensity to increase serum lipids, such as other PIs and the NRTI stavudine. In addition, all lipid parameters were collected in the fasted state, in contrast to some of the clinical trials[14] and cohort studies.[9-13] These data indicate that the serum lipid perturbations caused by the LPV/RTV combination are principally attributable to RTV.
These data are consistent with results from previous clinical trials and cohort studies demonstrating hyperlipidaemia when low-dose RTV is used to boost PIs other than LPV. For example, hyperlipidaemia was observed when RTV was used at a dosage of 100 mg twice daily to boost both indinavir 800 mg twice daily and saquinavir 1000 mg twice daily in the MaxCMin1 study.[17] Ritonavir, given at a dosage 200 mg once daily plus fosamprenavir 1400 mg once daily, was associated with higher rates of hypertriglyceridaemia than was NFV 1250 mg twice daily in the SOLO Study.[18] In the largest cohort study to date, the DAD Study,[11] the use of RTV alone or RTV in combination with other PIs (presumably RTV is used at low dosage in these combinations) was associated with significantly higher rates of elevated serum cholesterol and triglyceride levels, as well as reduced HDL cholesterol concentration, than regimens including NFV, indinavir or saquinavir without a second PI. We found no differences in the degree of RTV- or LPV/RTV-induced hyperlipidaemia between men and women, in contrast to the findings of Pernerstorfer-Schoen et al. who found a greater degree of hyperlipidaemia in women than in men on NFV.[19]
A potential limitation of this study is that it was conducted in HIV-negative subjects, as it is possible that the effects of RTV with or without LPV on serum lipids may differ in HIV-infected subjects. However, conducting this study in HIV-infected individuals would have run the risk of inducing antiretroviral resistance by using subtherapeutic concentrations of a single antiretroviral agent, and, consequently, we did not consider such a study design to be acceptable. The study was also restricted to subjects who were normolipidaemic at enrolment and it is possible that individuals with pre-existing hyperlipidaemia may experience a differential effect. In this regard, a small cohort study suggests that PI-induced hyperlipidaemia may be more pronounced in those with lower pretreatment serum lipid concentrations.[20]
The confirmation that RTV 100 mg twice daily causes adverse effects on serum lipids raises concerns about the practice of RTV boosting in a first antiretroviral regimen, when other options are available. It also suggests that doses of RTV lower than 200 mg daily should be investigated to determine whether these lower doses result in pharmacologically sufficient boosting of other PIs without the same degree of hyperlipidaemia, particularly as a correlation between the trough plasma level of RTV and hypercholesterolaemia has been demonstrated.[16] In this regard, atazanavir is currently being given with RTV 100 mg once daily, so that lower doses of RTV than 200 mg daily are already being used clinically.
However, the adverse effects of hyperlipidaemia need to be considered in the context of the proven benefits of PI-based HAART,[1-3] including the survival benefit of RTV in advanced HIV disease.[21] Some HIV-infected patients requiring HAART have low serum lipids and few or no risk factors for atherosclerosis. The use of a RTV-boosted PI regimen in such patients is not likely to be associated with a clinically significant increased risk of atherosclerotic disease. In addition, RTV-boosted PI regimens are an important component of 'salvage therapy' for patients who have failed to respond to other PI-based HAART,[22] and there are data emerging suggesting that double boosted PI therapy (low-dose RTV plus two other PIs) may be effective in 'deep salvage' of patients who have failed multiple previous antiretroviral regimens.[23] Antiretroviral drug-induced hyperlipidaemia may be more acceptable in salvage therapy than in initial therapy, given the more limited therapeutic options in salvage therapy, and the proven benefit of HAART which effectively suppresses HIV viraemia. Even so, antiretroviral drug-induced hyperlipidaemia is amenable to lipid-lowering therapy.[24]
References
1. Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338: 853-860.
2. Torres RA, Barr M. Impact of combination therapy for HIV infection on inpatient census. N Engl J Med 1997; 336: 1531-1533.
3. Ledergerber B, Telenti A, Egger M for the Swiss HIV Cohort Study. Risk of HIV related Kaposi's sarcoma and non-Hodgkin's lymphoma with potent antiretroviral therapy: prospective cohort study. Br Med J 1999; 319: 23-24.
4. Flexner C. HIV-protease inhibitors. N Engl J Med 1998; 338: 1281-1293.
5. The Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349: 1993-2003.
6. Danner SA, Carr A, Leonard JM et al. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med 1995; 333: 1528-1533.
7. Markowitz M, Saag M, Powderly WG et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med 1995; 333: 1534-1539.
8. Purnell JQ, Zambon A, Knopp RH et al. Effect of ritonavir on lipids and post-heparin lipase activities in normal subjects. AIDS 2000; 14: 51-57.
9. Periard D, Telenti A, Sudre P et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation 1999; 100: 700-705.
10. Calza L, Manfredi R, Farneti B, Chiodo F. Incidence of hyperlipidaemia in a cohort of 212 HIV-infected patients receiving a protease inhibitor-based antiretroviral therapy. Int J Antimicrob Agents 2003; 22: 54-59.
11. Pradier C, Sabin C, Friis-Moller N et al. Lipid profiles on therapy with PI. The DAD (Data Collection on Adverse Events of Anti-HIV Drugs) study. Sixth International Congress on Drug Therapy in HIV Infection. Glasgow, UK, November 2002 [Abstract PL12.1].
12. Manfredi R, Chiodo F. Disorders of lipid metabolism in patients with HIV disease treated with antiretroviral agents: frequency, relationship with administered drugs, and role of hypolipidaemic therapy with bezafibrate. J Infect 2001; 42: 181-188.
13. Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med 2000; 160: 2050-2056.
14. Walmsley S, Bernstein B, King M et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med 2002; 346: 2039-2046.
15. Gallant JE, Staszewski S, Pozniak AL et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. J Am Med Assoc 2004; 292: 191-201.
16. Gonzalez de Requena D, Blanco F, Garcia-Benayas T, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Correlation between lopinavir plasma levels and lipid abnormalities in patients taking lopinavir/ritonavir. AIDS Patient Care STDs 2003; 17: 443-445.
17. Dragsted UB, Gerstoft J, Pedersen C et al. Randomized trial to evaluate indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus type 1 infected patients: the maxCmin1 trial. J Infect Dis 2003; 188: 635-642.
18. Gathe JC Jr, Ive P, Wood R et al. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir/ritonavir versus twice-daily nelfinavir in naive HIV-1-infected patients. AIDS 2004; 18: 1529-1537.
19. Pernerstorfer-Schoen H, Jilma B, Perschler A et al. Sex differences in HAART-associated dyslipidaemia. AIDS 2001; 15: 725-734.
20. Segerer S, Bogner JR, Walli R, Loch O, Goebel F-D. Hyperlipidemia under treatment with proteinase inhibitors. Infection 1999; 27: 77-81.
21. Cameron DW, Heath-Chiozzi M, Danner S et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet 1998; 351: 536-537.
22. Loutfy M, Thompson C, Trpeski M et al. Virologic and immunologic responses of 292 ARV-experienced patients enrolled in the lopinavir/ritonavir expanded access program in Toronto, Canada. XIV. International Conference on AIDS. Barcelona, Spain, July 2002 [Abstract TuPeB4481].
23. Smith GHR, Klein MB, Murphy T et al. Double, boosted salvage therapy with lopinavir (LOP)/ritonavir (RIT) and saquinavir-sgc (SQR) in HIV-1 infected patients having failed 3 antiretroviral classes. XIV. International Conference on AIDS. Barcelona, Spain, July 2002 [Abstract TuPeB4547].
24. Dube MP, Stein JH, Aberg JA et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medicine Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis 2003; 37: 613-627.
|
|
| |
| |
|
|
|