| |
Cannabis Increased Risk for Psychosis Later in Life
|
| |
| |
STUDY (The Lancet July 28, 2007) AUTHORS CONCLUDED: We found a consistent increase in incidence of psychosis outcomes in people who had used cannabis. There was no statistical evidence of publication bias, although this finding was based on only seven studies. The pooled analysis revealed an increase in risk of psychosis of about 40% in participants who had ever used cannabis. However, studies tended to report larger effects for more frequent use, with most studies showing a 50-200% increase in risk for participants who used most heavily. A dose-response effect was observed in all studies that examined the relation to increasing cannabis exposure. Only three studies26,29,31 examined psychotic disorders as an outcome; the presence of functional impairment makes these studies relevant to clinical practice. The results from these studies were also consistent with an increased risk in people who used cannabis.... In conclusion, we have described a consistent association between cannabis use and psychotic symptoms, including disabling psychotic disorders. The possibility that this association results from confounding factors or bias cannot be ruled out, and these uncertainties are unlikely to be resolved in the near future. Despite the inevitable uncertainty, policymakers need to provide the public with advice about this widely used drug. We believe that there is now enough evidence to inform people that using cannabis could increase their risk of developing a psychotic illness later in life. The evidence that cannabis use leads to affective outcomes is less strong than for psychosis but is still of concern. Although individual lifetime risk of chronic psychotic disorders such as schizophrenia, even in people who use cannabis regularly, is likely to be low (less than 3%), cannabis use can be expected to have a substantial effect on psychotic disorders at a population level because exposure to this drug is so common."
Cannabis use and risk of psychosis in later life COMMENTARY
The Lancet July 28, 2007; 370:293-294
Published in this week's Lancet (see study results below) is the most comprehensive meta-analysis to date of a possible causal relation between cannabis use and psychotic and affective illness later in life.1 The authors conclude that the risk of psychosis increased by roughly 40% in people who have used cannabis, and that there is a dose-response effect, leading to an increased risk of 50-200% in the most frequent users.
The most important problems in studying the relation between cannabis use and psychosis are reverse causality and the transitory intoxication effect. If individuals with imminent psychotic disorder start to use cannabis to alleviate symptoms, the psychosis could be causing the cannabis use, rather than the other way around. In most of the studies included in the present meta-analysis, Theresa Moore and colleagues were able to adjust for the effect of psychotic or imminent psychotic symptoms and they were able to ensure that psychotic outcomes were not due to the transitory effect of intoxication. In observational studies, even the most thorough analysis cannot definitely rule out the possibility that confounding or bias can be responsible for the association between cannabis exposure and psychotic symptoms. However, in the present paper, the assessment of adjustment for confounding factors and transitory effects of cannabis intoxication is done more thoroughly than in previous reviews, and the odds ratio results for psychosis are more reliable and also more modest than seen in previous publications. We therefore agree with the authors' conclusion that there is now sufficient evidence to warn young people that cannabis use will increase their risk of psychosis later in life.
Current estimates of lifetime cannabis use among young adults in the UK are around 40%.2 If there is a true causal relation, the increased risk of 40% would mean that 14% of psychotic outcomes in the UK might not occur if cannabis was not used.3 This finding has tremendous implications for young people, their families, and society. Based on the results of a Danish study, we estimated the incidence rate of schizophrenia among 15-34 year olds to be around 37 per 100 000 person-years.4 With a 14% population attributable risk fraction, and around 15•5 million 15-34 year olds in the UK,5 this estimate means that around 800 yearly cases of schizophrenia in the UK could be prevented through cessation of cannabis consumption. Since more European countries are seeing an increase rather than a decrease in the prevalence of cannabis consumption, this finding is a cause for concern.6
The ultimate proof of a causal relation would be a large-scale placebo-controlled randomised trial of delta-9-tetrahydrocannabinol (the principal psychoactive component of cannabis) exposure in healthy young people with long-term follow-up. Since cannabis is illegal in most countries and its harmful effect on cognitive function is already well established,7,8 such a trial cannot be done because of practical and ethical reasons.However, there are a few small, short-term trials comparing cannabis and placebo in psychotic and non-psychotic people who were regular cannabis users. Until now, only two trials have been published9,10 that indicated that cannabis is responsible for transient exacerbation in psychotic core features. On the basis of such trials, the long-term effects could not be established, but the results indicate that there is at least a transitory psychotic effect, which theoretically correlates with the results of observational studies.
Results from both observational and experimental studies warrant investigation of whether dysfunction of the cannabinoid receptor system contributes to the pathophysiology of psychotic disorders in the range of schizophrenia.9
In the public debate, cannabis has been considered a more or less harmless drug compared with alcohol, central stimulants, and opioids. However, the potential long-term hazardous effects of cannabis with regard to psychosis seem to have been overlooked, and there is a need to warn the public of these dangers, as well as to establish treatment to help young frequent cannabis users.
Editorial: Rehashing the evidence on psychosis and cannabis
The Lancet July 28, 2007; 370:292
As cabinet ministers in the UK fall over themselves to tell all about their cannabis-taking younger days, Gordon Brown's Government begins its review of the classification of cannabis, with the probable outcome of relabelling it a class B drug of misuse. Possession would then become an offence likely to lead to arrest and perhaps a jail sentence. Cannabis was downgraded to class C in 2004, which meant that the penalties for possession, production, or supply were reduced.
The Advisory Council on the Misuse of Drugs will examine the evidence for harms caused by cannabis including those associated with the increasingly available strains such as skunk. In January, 2006, after its last review, the Advisory Council recommended that the class C status for cannabis should remain, but that resources should be put into education about the risks of cannabis and into further research on its effects on mental health.
As pointed out by Merete Nordentoft and Carsten Hjorthoj in a Comment, "published in this week's Lancet is the most comprehensive meta-analysis to date of a possible causal relation between cannabis use and psychotic and affective illness later in life". In their systematic review, Theresa Moore and colleagues found "an increase in risk of psychosis of about 40% in participants who had ever used cannabis", and a clear dose-response effect with an increased risk of 50-200% in the most frequent users.
In 1995, we began a Lancet editorial with the since much-quoted words: "The smoking of cannabis, even long term, is not harmful to health." Research published since 1995, including Moore's systematic review in this issue, leads us now to conclude that cannabis use could increase the risk of psychotic illness. Further research is needed on the effects of cannabis on affective disorders. The Advisory Council on the Misuse of Drugs will have plenty to consider. But whatever their eventual recommendation, governments would do well to invest in sustained and effective education campaigns on the risks to health of taking cannabis.
Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review
Theresa HM Moore MSc a, Dr Stanley Zammit PhD a c , Anne Lingford-Hughes PhD a, Thomas RE Barnes DSc d, Peter B Jones PhD e, Margaret Burke MSc b and Glyn Lewis PhD a
Summary
Background
Whether cannabis can cause psychotic or affective symptoms that persist beyond transient intoxication is unclear. We systematically reviewed the evidence pertaining to cannabis use and occurrence of psychotic or affective mental health outcomes.
Methods
We searched Medline, Embase, CINAHL, PsycINFO, ISI Web of Knowledge, ISI Proceedings, ZETOC, BIOSIS, LILACS, and MEDCARIB from their inception to September, 2006, searched reference lists of studies selected for inclusion, and contacted experts. Studies were included if longitudinal and population based. 35 studies from 4804 references were included. Data extraction and quality assessment were done independently and in duplicate.
Findings
There was an increased risk of any psychotic outcome in individuals who had ever used cannabis (pooled adjusted odds ratio=1•41, 95% CI 1•20-1•65). Findings were consistent with a dose-response effect, with greater risk in people who used cannabis most frequently (2•09, 1•54-2•84). Results of analyses restricted to studies of more clinically relevant psychotic disorders were similar. Depression, suicidal thoughts, and anxiety outcomes were examined separately. Findings for these outcomes were less consistent, and fewer attempts were made to address non-causal explanations, than for psychosis. A substantial confounding effect was present for both psychotic and affective outcomes.
Interpretation
The evidence is consistent with the view that cannabis increases risk of psychotic outcomes independently of confounding and transient intoxication effects, although evidence for affective outcomes is less strong. The uncertainty about whether cannabis causes psychosis is unlikely to be resolved by further longitudinal studies such as those reviewed here. However, we conclude that there is now sufficient evidence to warn young people that using cannabis could increase their risk of developing a psychotic illness later in life.
Introduction
Cannabis, or marijuana, is the most commonly used illegal substance in most countries, including the UK and USA.1-3 About 20% of young people now report use at least once per week or heavy use (use on >100 occasions).4,5 Use has increased particularly during early adolescence, when the developing brain might be especially susceptible to environmental exposures.6 Experimental studies7-10 and surveys of users11-13 provide strong evidence that cannabis intoxication can produce transient, and usually mild, psychotic and affective experiences. Of greater concern are chronic symptoms that persist beyond, or occur independently of, intoxication effects.
Whether cannabis increases the incidence of established syndromes such as schizophrenia or depression is unclear, but this question is important because these disorders lead to substantial distress for individuals and their families, and to public burden from health-care costs. Randomised controlled trials (RCTs) of cannabis for medical use14 are unlikely to be helpful in addressing the question of causality because there are substantial differences between the pharmacokinetic profiles of such preparations and of cannabis used as a recreational drug. The typically short follow-up periods of such trials also substantially hinder interpretation of results.
Previous reviews in this field have not been very systematic, have examined broad psychosocial outcomes rather than mental illness, or have included cross-sectional data.15-19 We have systematically reviewed longitudinal studies of cannabis use and subsequent psychotic or affective mental health outcomes, and we have assessed the strength of evidence that cannabis use and these outcomes are causally related.
Methods
Study selection and data collection
Studies were included if they were population-based longitudinal studies, or case-control studies nested within longitudinal designs. We excluded cohorts of people with mental illness or substance-use-related problems, studies of prison populations, and RCTs of cannabis for medical use.14
Diagnostic outcomes for psychosis included schizophrenia, schizophreniform, schizoaffective, or psychotic disorders, non-affective or affective psychoses, psychosis not otherwise specified, psychotic symptoms, delusions, hallucinations, or thought disorder. Presence of delusions, hallucinations, or thought disorder was a requirement for all psychosis outcomes. Affective, mood, or bipolar disorder, affective disorder not otherwise specified, depression, suicidal ideation or suicide attempts, anxiety, neurosis, and mania were included for affective outcomes.
We searched the following databases from their inception to Sept 5, 2006: Medline, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) on OVID; PsycINFO on WebSPIRS; ISI Web of Knowledge and ISI Proceedings; ZETOC (a British library database of journal and conference contents); BIOSIS on EDINA; and Latin American and Caribbean Health Sciences (LILACS) and Caribbean Health Sciences Literature (MEDCARIB). We searched using the format "([psychosis or schizophrenia or synonyms] or [affective disorder or depression or synonyms]) and (cannabis or synonyms)", using text words and indexing (MeSH) terms (full details are available on GL's departmental website).
The search was restricted to studies on human beings but was not limited by language or study design. We searched reference lists of included studies, and wrote to experts in the field and researchers responsible for studies to find other published and unpublished studies of relevance. We examined all titles and abstracts, and obtained full texts of potentially relevant papers. Working independently and in duplicate, we read the papers and determined whether they met inclusion criteria using eligibility record forms (available on the corresponding author's departmental website). We resolved disagreements by consensus, and extracted data independently and in duplicate.
We assessed study quality by recording how potential non-causal explanations, particularly bias and confounding factors, were accounted for in each study. We assessed information on sampling strategy, response rates, missing data, attrition, and attempts to address reverse causation, intoxication effects, and confounding factors.
Data synthesis
Where study characteristics were judged reasonably homogeneous, we grouped studies together and pooled data in a meta-analysis; otherwise, we present a narrative synthesis of data. We pooled studies using the DerSimonian and Laird random-effects model20 and the metan command in Stata (9•0). Where studies presented data only in subgroups, these were incorporated as separate studies. We assessed heterogeneity using the I2 statistic.21 Presence of publication bias was investigated by use of funnel plots and Egger's Test.22 A summary of compliance with MOOSE guidelines23 is available on GL's departmental website.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Searches of electronic bibliographic databases, expert advice, and searches of reference lists of included studies and other reviews yielded 4804 references. On the basis of their titles and abstracts, we judged that 175 (3•6%) of these references potentially contained enough detail to be relevant. 143 of these references were excluded as not relevant when we had read the whole paper. Details of the studies that were excluded at this stage, including those that we regarded as near misses, are available on GL's departmental website.
We found 11 studies of psychosis; these reports presented data from seven cohort studies. There were five adult population-based cohorts: the Epidemiological Catchment Area (ECA) study based in the USA;24 the Early Developmental Stages of Psychopathology (EDSP) study based in Germany;25 the Netherlands Mental Health Survey and Incidence Study (NEMESIS);26 the National Psychiatric Morbidity Survey (NPMS) based in the UK;27 and the 1969 Swedish Conscript Cohort.28-30 There were also two birth cohorts, from Dunedin31,32 and Christchurch (CHDS)33,34 in New Zealand. For the Swedish conscripts and CHDS cohorts, data from the most recent reports29,34 were included in each case because these had longer follow-up to cover more events, and had more comprehensive analyses to keep reverse causation and confounding effects to a minimum. Omission of individuals with schizophrenia simplex made no difference to results for schizophrenia in the study of Swedish conscripts (Zammit S, unpublished). However, results for non-schizophrenia psychoses from this cohort were not included because the diagnostic codes that were used potentially included many people without psychosis as defined in this study.
Three of the eligible studies examined psychotic disorders, which were defined as the presence of psychotic symptoms with concurrent evidence of impaired functioning (Dunedin,31 NEMESIS,26 and Swedish conscripts29), and six studies used the broader outcome of psychotic symptoms with no requirement for impaired functioning (CHDS,34 Dunedin,31 ECA,24 EDSP,25 NEMESIS,26 and NPMS27).
For affective outcomes, 24 reports were identified from 15 cohort studies: two birth cohorts from New Zealand (CHDS35-37 and Dunedin31,32,38); six adult population-based cohorts, from the USA (Berkely,39 ECA,40,41 and NY state42,43), the UK (NPMS [Haynes J, University of Bristol, personal communication]), Australia (Northern Rivers Mental Health Study, NoRMHS44), and Colombia;45 and seven school-based cohorts, from Australia (Victoria46) and the USA (AddHealth,47,48 Baltimore,49 Chicago,50 LA schools,51,52 LAT,53 and NY schools54,55). Various outcomes were examined, including depression (ten studies), depressive symptoms (six studies), suicidal ideation or suicide attempts (six studies), anxiety disorders (five studies), and anxiety symptoms (one study). We identified one study that had data on mania, though there was only one event in the whole sample.44
Results for the seven studies included for psychosis are summarised in webtable 1 and figure 1. There was no evidence to support the presence of publication bias (Egger test, p=0•48). The unadjusted results of all studies reported evidence of an increased risk of psychosis in people who used cannabis compared with non-users. These associations were reduced, but nevertheless persisted, in six of the studies after adjustment for confounding factors.
Figure 1. Forest plot showing adjusted odds ratios and 95% CI for any psychosis outcome according to ever use of cannabis in individual studies
Exposure was ever use of cannabis in all studies except for the NPMS, in which the measure was ever use over the past 1 year only. *Additional data were provided by investigators in these studies.31,34 Results were unaltered when the 4% of cases with simplex schizophrenia were omitted.
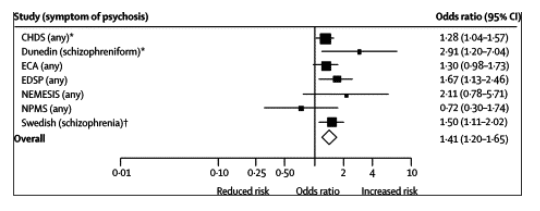
Estimates were pooled under the assumption that measures of psychosis were on a continuum of symptoms from mild (self-report of psychotic symptoms) to severe (clinical diagnosis of schizophrenia). There was an increased risk of a psychotic outcome in individuals who ever used cannabis (adjusted odds ratio=1•41, 95% CI 1•20-1•65; heterogeneity p=0•28; I2=19•2%).
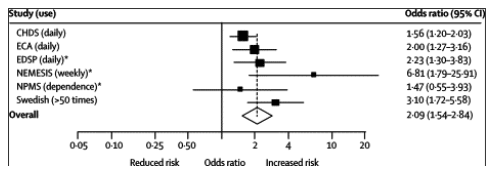
Of the six studies that either examined a linear trend across cannabis use frequencies25,26,29,34 or compared higher with lower frequency categories,24,27 all reported findings that were consistent with a dose-response effect. Figure 2 shows the associations reported for people with most frequent cannabis use compared with non-users of cannabis in each study where such data were available. In the pooled analysis, there was an increased risk of a psychotic outcome in individuals who used cannabis most frequently (adjusted odds ratio=2•09, 1•54-2•84; heterogeneity p=0•11; I2=44•1%).
Figure 2. Forest plot showing adjusted odds ratios and 95% CI for any psychosis outcome according to most frequent use of cannabis in individual studies
*Results were not adjusted for other drug use.
We also examined the specific relation between cannabis use and risk of developing a psychotic disorder. The narrowest definition of psychotic disorder was in the Swedish conscripts study,29 which reported an increased risk of schizophrenia in individuals who used cannabis. Studies of schizophreniform disorder from Dunedin31 (additional data unstratified by age at first use were supplied by the researchers) and needs-based diagnosis of psychotic disorder from NEMESIS26 also showed associations with cannabis use. Pooled data from these studies show an increased risk of psychotic disorders in individuals who had ever used cannabis (adjusted odds ratio=2•58, 1•08-6•13; heterogeneity p=0•049; I2=66•9%).
We repeated the pooled analyses for most frequent cannabis use and for psychotic disorders but omitted the results from NEMESIS because this had the greatest effect on heterogeneity. In these sensitivity analyses, there remained an increased risk of a psychotic outcome in people who used cannabis most frequently (odds ratio=1•92, 1•50-2•47; heterogeneity p=0•26; I2=25•0%), and also an increased risk of psychotic disorders in people who had ever used cannabis (odds ratio=1•82, 1•01-3•30; heterogeneity p=0•16; I2=48•3%).
Two studies have examined differential effects of cannabis on psychosis according to age of first use of this drug. In the Dunedin study, a stronger effect of cannabis on psychotic symptoms was reported for individuals who first used cannabis before, as opposed to after, 16 years of age.31 There was much weaker evidence for this age effect for schizophreniform disorder, although the CIs were very wide. In the Swedish conscripts study, there was no evidence that the effect of cannabis on risk of schizophrenia differed for people who first used cannabis before, as opposed to after, age 16 years.30
Other putative interactions were also reported. A further report on the Dunedin cohort32 described a strong effect of cannabis on risk of schizophreniform disorder in people homozygous for the valine allele at Val158Met within the catechol-O-methyltransferase (COMT) gene (crude odds ratio=10•9, 2•2-54•1), with no apparent effect in methionine homozygotes (crude odds ratio=1•1, 0•21-5•4) and an intermediate effect in heterozygotes. This potential interaction was observed only in people who first used cannabis before age 18 years, with no evidence of interaction in those who first used it after this age.
In the EDSP study, the effect of cannabis on psychosis outcome was stronger in groups described as psychosis prone than in non-prone groups.25 However, psychosis-prone individuals already had evidence of psychotic features at baseline, and this study was therefore not examining differences in the effects of cannabis on psychosis incidence between these groups.
We assessed the quality of the studies included for psychosis. Because reverse causation, intoxication effects, and confounding factors could have led to overestimation of the true causal association between cannabis use and psychosis, we assessed the degree to which the potential effect of these was kept to a minimum within each study (table 1).
Four studies24,26,27,29 excluded participants who had experienced psychosis at baseline. In the Swedish conscripts study,29 reverse causation was limited further by analysis restricted to patients admitted for schizophrenia at least 5 years after conscription; this analysis produced similar results to the main analysis. Three studies25,31,34 adjusted in the analysis for psychotic symptoms at baseline. Although this approach partly addresses the problem of reverse causation, it averages the association between cannabis and psychosis incidence with that between cannabis and symptom chronicity or relapse. In CHDS,34 structural equation modelling results suggested that cannabis use was significantly associated with subsequent increase in risk of psychotic symptoms rather than vice versa.
Intoxication effects were not specifically mentioned in reports of the ECA24 and NEMESIS26 studies, but the outcome assessment (also used in Dunedin31 and EDSP25) instructs the interviewer to exclude psychotic symptoms that arise solely from drug use. The questionnaires used in CHDS34 and NPMS27 do not allow intoxication to be assessed. However, exclusion of intoxication effects in those who use cannabis every day is likely to be very difficult. In the Swedish conscripts study,29 use of WHO International Classification of Diseases criteria suggests that misclassification of a cannabis-intoxication psychosis was unlikely; the same is probably true for the Dunedin study,31 in which psychosis was defined by the presence of symptoms of schizophreniform disorder for longer than 1 month.
The studies listed in table 1 adjusted for about 60 different confounding factors, including other substance use, personality traits, sociodemographic markers, intellectual ability, and other mental health problems. For all studies, fully adjusted estimates were attenuated, compared with crude results, by an average of about 45% (range 10%-80%). In the CHDS, use of fixed-effects regression to adjust further for unmeasured non-varying confounding factors made little difference to results.34 Adjustment for other substance use led to a substantial attenuation of effect in the ECA study24 and NEMESIS,26 whereas in the Swedish conscripts study29 the strongest confounding factors were IQ score, urban upbringing, and other mental health disorders.
Loss to follow-up occurred for between 4%31 and 32%27 of the cohorts we included for psychosis. No data on attrition were available from the Swedish conscript study.29 Sensitivity analyses from two of the studies26,34 suggest that attrition might have had small effects on results, although reclassification of outcome in NEMESIS did not differentiate between people with different cannabis use at baseline.
Depressive outcomes were examined in 15 cohorts (webtable 2). There was no evidence to support the presence of publication bias (Egger test, ten studies; p=0•13). Of ten studies that examined a diagnosis of depression or above-threshold rating scores (figure 3), five reported evidence of an association with cannabis use that persisted after adjustment.31,37,40,43,46 However, in two of these,31,46 significant associations were observed only in subgroup analyses, and in the Dunedin study31 no baseline measures of depression were accounted for. Weak evidence for association was reported by two further studies,45,48 and an association observed in NPMS (Haynes J, personal communication) was eliminated after adjustment. In view of the heterogeneity across these studies in relation to measures of cannabis exposure (which included ever use, frequency of use, and cannabis misuse disorder), we did not think a meta-analysis of these data would be appropriate. Using average values from these studies-of 35% having ever used cannabis and 15% having developed depression-we estimated that a sample with more than 230 events would be required for 80% power to detect an odds ratio for depression of 1•5 (a larger effect than was observed in most studies). Thus, about half the studies probably had insufficient power to observe an association of this size.
Figure 3. Forest plot showing adjusted odds ratios and 95% CI for any depression outcome according to cannabis exposure in individual studies
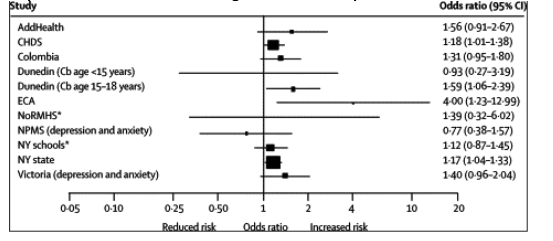
Six studies32,38,51-54 from five cohorts examined depressive symptoms on a continuous scale. Evidence of association was observed in the Berkeley38 and LA schools51,52 cohorts (webtable 2). However, in the Berkeley study only crude results were presented, and the association was observed in men but not women,39 whereas in the LA schools cohort an increased risk observed in an early part of the study51 was not replicated in a later wave.52
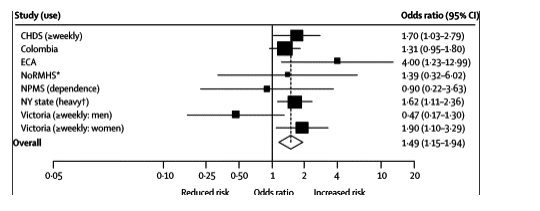
Of the studies that examined a linear trend across cannabis use frequencies37,41,51,52 or that compared higher with lower frequency groups46 (Haynes J, personal communication), four reported findings that were consistent with a dose-response effect on depression outcomes (webtable 2). Figure 4 shows the associations reported for participants with most frequent cannabis use compared with non-users, with some evidence for an increased risk of depression in a pooled analysis (adjusted odds ratio=1•49, 1•15-1•94; heterogeneity p=0•192; I2=29•6%).
Figure 4. Forest plot showing adjusted odds ratios and 95% CI for depression outcomes according to most frequent use of cannabis in individual studies
Depression outcome measures: DSM diagnosis (CHDS, NY state); ICD-10 diagnosis (NoRMHS); DSM symptom lasting ≥2 weeks (ECA); CIS-R score ≥12 (NPMS, Victoria); and SCL-90 upper quartile (Colombia). Subgroup data from the Victoria study were incorporated as separate studies. *Unadjusted results calculated from data in tables in the original study.44 Results for the heavy use category were calculated from results for linear trend across four categories of frequency of cannabis use.
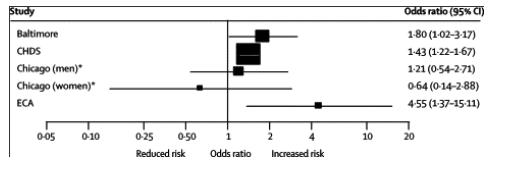
Seven studies assessed suicidal ideation or suicide attempts. Four of these10,37,47,49 reported an association between cannabis use and increased risk in adjusted analyses and one50 showed little evidence of an association (figure 5). A reduced risk of attempts51 but increased risk of ideation52 were reported from the LA schools cohort (webtable 2).
Figure 5. Forest plot showing adjusted odds ratios and 95% CI for suicidal ideation according to cannabis exposure in individual studies
Cannabis exposure: ever used before age 16 (Baltimore); used >40 occasions (Chicago); frequency of use (linear trend across frequency categories; CHDS); cannabis misuse disorder (ECA). *Unadjusted results; subgroup data incorporated as separate studies.
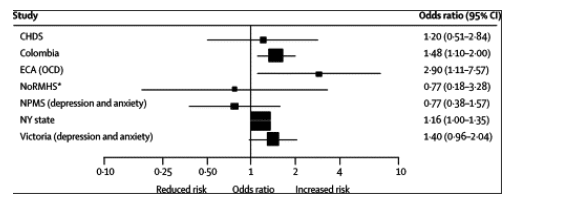
Of the seven studies that specifically examined anxiety outcomes (figure 6),35,41,42,44,45,51,52 two reported an association with cannabis use that persisted after adjustment for confounding factors.42,45 In the ECA study of obsessive-compulsive disorder, an association was observed in a matched sample, but there was little evidence for association in the whole sample when a more valid unconditional analytical approach was used.41
Figure 6. Forest plot showing adjusted odds ratios and 95% CI for anxiety outcomes according to cannabis exposure in individual studies
Anxiety outcomes: DSM diagnosis (CHDS, ECA, NY state); ICD-10 diagnosis (NoRMHS); CIS-R score ≥12 (NPMS, Victoria); SCL-90 upper quartile (Colombia). Cannabis exposure: use in past year (ECA, NPMS); use less than once per week for the past 6 months (Victoria); use at least once per month (Colombia); frequency of use (linear trend across frequency categories; CHDS, NY state); cannabis disorder (NoRMHS). OCD=obsessive-compulsive disorder. *Unadjusted results calculated from data in tables in the original study.44
Several studies reported putative interactions. In the NY state study,43 there was a suggestion that risk of depression increased with earlier age of first use of cannabis. However, in the Dunedin cohort, there was no evidence for a greater risk of depression in people who first used cannabis before, as opposed to after, age 16 years.31
There were single reports of interactions between cannabis use and age37 and sex.46 As with the results for psychosis, an interaction between cannabis use and COMT genotype was observed in the Dunedin cohort for depression, but not for anxiety.32
We assessed the quality of the studies included for affective outcomes by assessing the degree to which the potential effects of reverse causation, intoxication effects, and confounding factors were kept to a minimum within each study (table 2). Only four studies40,41,48 (Haynes J, personal communication) excluded participants with affective symptoms at baseline. Ten reports35,37,38,42,43,45,46,49,51,55 adjusted for the baseline measure of the outcome in the analyses but, as discussed earlier, this potentially mixes the effects of cannabis on incidence of affective outcome with those on symptom chronicity or relapse. In seven studies,31,39,44,47,50,52,53 there was no exclusion of affected individuals or adjustment for baseline measures in the analyses. Three papers42,43,45 from two cohorts reported that adjustment for baseline measures of the outcome had a negligible effect on results. Attempts to exclude intoxication effects were not explicitly mentioned in any of the 24 studies, although the questionnaires and interviews used for ten of these studies could have enabled raters to exclude symptoms attributed to drug intoxication.
About 50 different confounding factors were reported. Most of these were related to family and peer relationships, adverse life events, criminality, mental health problems, sociodemographic markers, and other substance use. Five studies39,44,50,53,54 presented only unadjusted results, whereas one55 made no mention of the confounding factors adjusted for.
For six of the eight studies in which both crude and adjusted results were presented, adjustment led to attenuation of associations with cannabis use, ranging from 10% to 100% reduction. Adjustment for a comprehensive set of confounding factors in the CHDS eliminated reasonably strong associations with depression, anxiety, and suicidal ideation,35 although associations for depression persisted after adjustment in a longer follow-up of this cohort.37 In one study of the Dunedin cohort,38 adjustment for a more comprehensive set of confounding factors than in a more recent report31 reduced the association between cannabis use and all mental disorders by 90%; however, effects specifically for affective outcomes were not presented. In the Baltimore study, both an increase in effect size for suicidal ideation and an attenuation of association with suicide attempts were observed following adjustment.49
Attrition from the studies included for affective outcomes ranged from 4%31 to 70%,51 with a median of 20%; loss to follow-up was not reported for two of the studies.39,45 Cannabis use disorder at baseline was associated with increased attrition in NoRMHS, although weighting of the analyses to account for this made little difference to the results.44 Attrition was associated with baseline alcohol use and disorganised thinking in the LA schools study,52 but was not associated with baseline substance use in the LAT study.53 However, in the ECA study individuals with baseline depression and cannabis misuse were both more likely to be available for follow-up.40
Discussion
We found a consistent increase in incidence of psychosis outcomes in people who had used cannabis. There was no statistical evidence of publication bias, although this finding was based on only seven studies. The pooled analysis revealed an increase in risk of psychosis of about 40% in participants who had ever used cannabis. However, studies tended to report larger effects for more frequent use, with most studies showing a 50-200% increase in risk for participants who used most heavily. A dose-response effect was observed in all studies that examined the relation to increasing cannabis exposure. Only three studies26,29,31 examined psychotic disorders as an outcome; the presence of functional impairment makes these studies relevant to clinical practice. The results from these studies were also consistent with an increased risk in people who used cannabis.
Studies included in the pooled analyses used different methods to measure cannabis exposure and to assess outcome. For example, use of the symptom checklist 90 assessment in the CHDS34 might have led to inclusion of participants without psychotic symptoms as defined in this review. This heterogeneity was reflected in the large I2 values for some of the pooled results. The features of NEMESIS26 that caused this study to increase between-study heterogeneity to a greater extent than the other studies are not clear, but heterogeneity decreased when this study was omitted from the sensitivity analyses, even though results were largely unchanged.
Arguments for why earlier use of cannabis might have more harmful effects are intuitively compelling, but no robust evidence supports this view. The increased risk of psychosis in people using cannabis from a younger age observed in the Dunedin cohort could indicate a greater cumulative exposure to cannabis rather than a sensitive period of exposure.31 In the Swedish conscripts study, in which cumulative use of cannabis was examined, no difference in risk according to age at first use was observed.30 Similarly, evidence for effect modification between cannabis use and COMT variation on psychosis risk is very weak: this effect was observed in only a subgroup of people within the Dunedin cohort,32 and evidence for such an interaction in an experimental setting was also observed in only a subgroup of participants.56
Almost all studies reported an increased risk of affective outcomes in people who used cannabis, although CIs were generally consistent with null effects. However, effect sizes were small, and many studies were probably underpowered. For example, odds ratios for depression ranged from 1•3 to 1•6 for the highest exposure categories of weekly or monthly cannabis use, with one exception (the ECA study of cannabis misuse disorder).40
An association seen in an observational study does not necessarily reflect a causal relation. Because most of the studies for psychosis excluded people with psychosis at baseline, the observed associations are unlikely to reflect reverse causation. However, the majority of studies for affective outcomes did not adequately address the problem of reverse causation as a possible alternative explanation for any association observed. For cannabis and psychosis, there was evidence of confounding effects, but the associations persisted in almost all studies, even after adjustment for comprehensive lists of variables, including markers of premorbid disturbances that are commonly observed in patients with schizophrenia. All of the studies that reported an association for psychosis adjusted for other drug use, although two of the studies26,31 made no adjustment for alcohol use. Furthermore, three studies25,26,31 for psychosis made no adjustment for other mental health disorders at baseline, and measures of disorders adjusted for in the other studies24,25,29,34 are unlikely to have accurately captured all mental health symptoms at baseline given the scope of the assessment tools generally used.
Residual confounding by these or other factors can never be eliminated from observational studies. Adjustment for confounding factors in studies of affective outcomes seemed to be more important than in studies of psychotic outcomes, and in some studies such adjustment explained all the observed association. There was also more variation for affective outcomes than for psychosis, with increases in crude estimates reported in two studies.41,49 Furthermore, roughly half the studies made no adjustment for alcohol or other drug use. Confounding factors seem more likely to explain the reported association between cannabis and affective outcomes than that between cannabis and psychosis.
Most studies of psychosis made some attempt to reduce the chance that the outcome examined was due directly to effect of intoxication with cannabis, although this can be a difficult judgment in people who use cannabis frequently. Misdiagnosis as cannabis intoxication was unlikely in the Swedish conscripts study,29 in which the outcome was admission to hospital with schizophrenia, or in the Dunedin cohort,31 in which the outcome was presence of schizophreniform symptoms for longer than 1 month. The possibilities of intoxication and withdrawal effects were not considered in any of the studies of affective outcomes, although both of these can result from cannabis consumption.7,57
We would expect both confounding factors and intoxication to lead to an increase in the observed association. However, underestimation of effects could also have occurred. Measurement of cannabis use is especially difficult because there is almost certainly large variation in biologically available cannabinoid concentrations, resulting from different sources of cannabis and from different intake practices; self-reported frequency of use is also prone to error. Such misclassification, if random, would usually make detection of an association more difficult. However, differential misclassification could lead to overestimates of association. For example, stimulant use is more common in people who use cannabis than in those who do not, so under-reporting of stimulant use might differentially affect the results from cannabis users.
Attrition in cohort studies is more likely in people who use drugs and in those who develop mental health problems than in other participants,58,59 and this would also lead to underestimates of association. Evidence for such a pattern of attrition was present in NoRMHS44 and the LA schools study.52 In the ECA study,40 participants with baseline depression were more likely to remain in the study, although pattern of loss in relation to incident depression is unknown. The extent to which such bias would affect the results is unclear, although modelling for attrition in CHDS,34 NEMESIS,26 and NoRMHS44 suggests that bias caused by attrition had little effect on the overall findings.
Recent estimates of the proportion of adolescents and young adults in the UK who have ever used cannabis are around 40%.2 If having ever used cannabis increases risk of a psychotic outcome by 1•4 times (as suggested from the pooled analysis), we can estimate that about 14% (95% CI 7-19) of psychotic outcomes in young adults currently in the UK would not occur if cannabis were not consumed. However, such estimates rely heavily on the assumption that the association between cannabis use and psychosis is causal, and that the pooled relative risk is an accurate estimate of this causal effect.
Projected trends for schizophrenia incidence have not paralleled trends in cannabis use over time, and this apparent mismatch has been used as an argument against causal effects.60 However, other projections suggest that time lags and a lack of reliable incidence data might mean that changes in schizophrenia incidence are not yet fully apparent.61
Even seemingly robust findings from observational studies have sometimes not been confirmed by RCTs.62 However, in some situations RCTs are not feasible, and reliance must be placed on interpretation of results from the best available evidence from observational studies.63 The neurobiological sequelae of cannabis use, including modulated activity of dopaminergic, GABAergic, and glutamatergic neurons,64-66 are consistent with abnormalities described in people with psychotic disorders.67 Furthermore, evidence that cannabis can produce transient psychotic and mood-altering symptoms in experimental studies7-10 lends support to a causal explanation for the associations between cannabis use and more chronic psychotic and affective disorders.
We are not aware of any other systematic reviews that focus on the relation between cannabis and affective outcomes. A previous systematic review of cannabis use and psychosis included cross-sectional studies and did not address study quality.19 Another systematic review examined broader psychosocial outcomes,18 but the lack of focus specifically on psychotic or affective disorders meant that the explanations for associations could not be examined in detail. Previous meta-analyses from both systematic19 and narrative15,17 reviews (table 3) have included cross-sectional data17,19 or used unadjusted results,19 and combined effects for ever-use of cannabis with those for dependence.15,17,19 As might be expected, all report larger effects than observed in this study, although direct comparison of these effects with our findings is difficult.
There are potential problems with meta-analyses of observational data.68 However, we applied robust methods to identify as many publications as we could, and attempted to interpret the findings as appropriately as possible by including a thorough critique of individual studies, and by doing a comprehensive assessment of alternative explanations for associations reported.
Even if the methods of future longitudinal studies are more robust, these studies are likely to encounter similar limitations to those discussed here. However, improvement in the measurement of cannabis exposure and elimination of intoxication effects might reduce some of the uncertainty. Animal models of long-term effects of cannabis on neuropsychological domains relevant to psychotic or affective states could also improve knowledge.69 Further study is needed to establish whether cannabis is more harmful in younger age groups, and whether risk is modified by genetic or other factors. The question of whether cannabis causes psychotic or affective disorders is perhaps the wrong one to be asking, because it will be difficult to answer with any degree of certainty. What is more pertinent is whether the evidence that is now available can justify policy implications, such as public education campaigns to alert people to the possible risks associated with cannabis.
In conclusion, we have described a consistent association between cannabis use and psychotic symptoms, including disabling psychotic disorders. The possibility that this association results from confounding factors or bias cannot be ruled out, and these uncertainties are unlikely to be resolved in the near future. Despite the inevitable uncertainty, policymakers need to provide the public with advice about this widely used drug. We believe that there is now enough evidence to inform people that using cannabis could increase their risk of developing a psychotic illness later in life. The evidence that cannabis use leads to affective outcomes is less strong than for psychosis but is still of concern. Although individual lifetime risk of chronic psychotic disorders such as schizophrenia, even in people who use cannabis regularly, is likely to be low (less than 3%), cannabis use can be expected to have a substantial effect on psychotic disorders at a population level because exposure to this drug is so common.
|
|
| |
| |
|
|
|