| |
Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials
|
| |
| |
The Lancet Oct 2 2007; 370:1129-1136
Rodrigo M Lago MD a, Premranjan P Singh MD a and Richard W Nesto MD
a. Lahey Clinic Medical Center, Burlington, MA, USA
RWN is on the Speaker's Bureau and is involved in research funded by Glaxo Smith Kline and Takeda. RML and PPS declare no conflicts of interest.
Summary
Background
The overall clinical benefit of thiazolidinediones (TZDs) as a treatment for hyperglycaemia can be difficult to assess because of the risk of congestive heart failure due to TZD-related fluid retention. Since prediabetic and diabetic patients are at high cardiovascular risk, the outcome and natural history of such risks need to be better understood. We aimed to examine the risk of congestive heart failure and of cardiac death in patients given TZDs.
Methods
We used a search strategy to identify 3048 studies. 3041 were excluded, and we did a systematic review and meta-analysis of the seven remaining randomised double-blind clinical trials of drug-related congestive heart failure in patients given TZDs (either rosiglitazone or pioglitazone). We calculated pooled random-effects estimates of the risk ratios for development of congestive heart failure in patients given TZDs compared with controls. The main outcome measures were development of congestive heart failure and the risk of cardiovascular death.
Findings
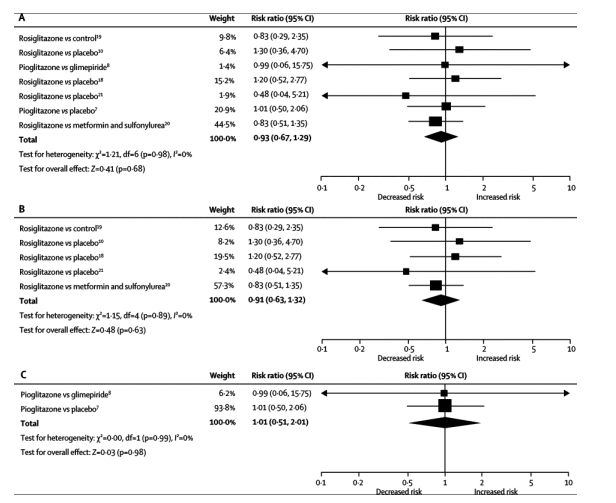
360 of 20191 patients who had either prediabetes or type 2 diabetes had congestive heart failure events (214 with TZDs and 146 with comparators). Results showed no heterogeneity of effects across studies (I2=22•8%; p for interaction=0•26), which indicated a class effect for TZDs. Compared with controls, patients given TZDs had increased risk for development of congestive heart failure across a wide background of cardiac risk (relative risk [RR] 1•72, 95% CI 1•21-2•42, p=0•002). By contrast, the risk of cardiovascular death was not increased with either of the two TZDs (0•93, 0•67-1•29, p=0•68).
Introduction
The prevalence of type 2 diabetes is increasing, and mortality from cardiovascular disease is two-fold to eight-fold higher in people with diabetes than in those without.1 Thiazolidinediones (TZDs) are synthetic selective ligands of the nuclear transcription factor, peroxisome-proliferator-activated receptor ϒ (PPARϒ). TZDs enhance insulin sensitivity2,3 and are effective agents for control of glycaemia in patients with type 2 diabetes. Both rosiglitazone and pioglitazone have been shown to have a positive cardiometabolic profile that is independent of their effects on glycaemia.4-6 One trial7 showed that pioglitazone reduced major cardiovascular events in patients with type 2 diabetes; another8 that pioglitazone slowed progression of carotid artery intima-media thickening. However, a recent meta-analysis has questioned the cardiac safety of rosiglitazone.9
The clinical use of TZDs has been limited by the fact that they cause fluid retention, and therefore could potentially lead to development of congestive heart failure in patients with or without pre-existing left ventricular systolic or diastolic dysfunction. However, TZDs do not directly affect left ventricular systolic or diastolic function.10,11 In 2003, the American Heart Association and American Diabetes Association (AHAADA) issued a consensus statement on the issue of congestive heart failure and provided guidelines for use of TZDs in patients with type 2 diabetes, with or without coexisting cardiovascular disease.12
The overall clinical benefit from use of TZDs in randomised clinical trials might be difficult to gauge because of the risk of congestive heart failure. Moreover, the outcome and natural history of congestive heart failure that is caused by TZD-related fluid retention has not been defined. TZD-related congestive heart failure could either be a drug-related adverse event or a negative outcome that might ultimately affect the survival of patients who receive TZDs. We did a systematic review and meta-analysis of pooled data from randomised trials of TZDs in subjects with prediabetes or type 2 diabetes to assess the risk of development of heart failure and death from cardiovascular causes in patients given TZDs.
Results
We identified 3048 publications from our systematic review. A preliminary review of these studies led us to reject 2387 of them: 634 because they were not original and 1753 because they were not relevant to our aim. Of the 661 remaining studies, 654 were excluded because they either did not randomise patients in their study design (n=394) or did not measure or report outcome data for congestive heart failure or death (n=260). Ultimately, the meta-analysis included seven studies7,8,10,18-21 including one with two control groups.19Table 1 summarises the characteristics of these studies and their participants. All studies had been published since 2005 and followed up patients for between 12 and 48 months, with a mean of 29•7 months. Trial populations ranged from 200 to 5269 participants, with a median of 4351.
The definition of congestive heart failure varied between trials, but in general consisted of investigator-reported or adjudicated congestive heart failure events that required admission to hospital (table 1). Although one study7 also reported events that did not require admission to hospital, we chose to exclude those events from analysis, to be consistent with the level of certainty of the diagnosis and severity of congestive heart failure reported in most other studies.
In the seven randomised clinical trials, 360 congestive heart failure events were reported in 20 191 participants with prediabetes or type 2 diabetes (214 in the 9360 patients who were given TZDs, and 146/10 831 were given comparators). The age of the participants ranged from 54•7 to 64 years, with a mean of 59•4; 83% were white, and 64•8% were men.
Results showed no heterogeneity of effects across studies (I2=22•8%, p=0•26), which indicated a class effect for TZDs. The observed inconsistency suggested that most of the variability between studies was due to chance. Figure 2 shows that, compared with controls, patients given TZDs had increased risk of congestive heart failure (RR 1•72, 95% CI 1•21-2•42, p=0•002). Figure 3 shows that, by contrast, the risk of cardiovascular death was not significantly increased with the use of either rosiglitazone or pioglitazone, compared with controls (0•93, 95% CI 0•67-1•29, p=0•68).
Figure 2. Overall risk for congestive heart failure with (A) TZDs; (B) rosiglitazone; and (C) pioglitazone
TZD= thiazolidinediones. Forest plot of risk ratios (RR) on a logarithmic scale for trials pooled with Mantel-Haenszel weighting. The area of each square is proportional to the weight of the corresponding study, measured as the inverse of the estimated variance of the log risk ratio. The diamond represents the pooled relative risk, and its width represents its 95% CI. A horizontal line represents each study, with its effect size and 95% CIs. The solid vertical line corresponds to no risk. df=degrees of freedom.
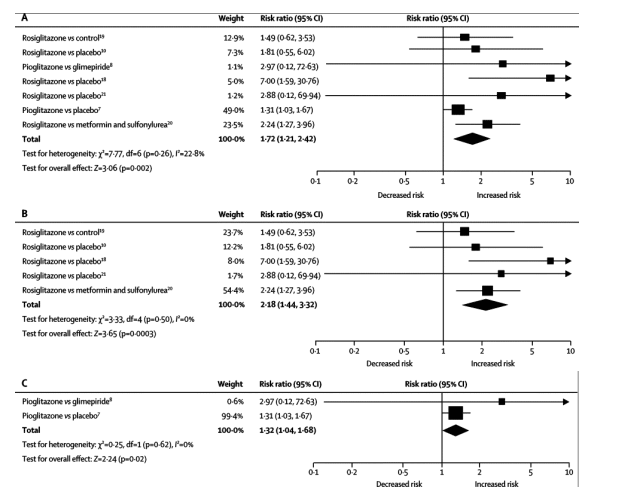
Figure 3. Overall risk for cardiovascular death with (A) TZDs; (B) rosiglitazone; and (C) pioglitazone trials
TZDs=thiazolidinediones. Risk ratios (RR) are shown on a logarithmic scale, the size of each square is proportional to the weight of the individual study, measured as the inverse of the estimated variance of the log risk ratio. The diamond represents the pooled relative risk and its width represents its 95% CI. df=degrees of freedom.
The overall event rate for congestive heart failure was 2•3% in patients in the TZD group and 1•4% in the comparator group. The overall event rate for cardiovascular death was 0•7% in both groups. Furthermore, the overall attributable event rate for congestive heart failure was 0•9% per year in the TZD group and 0•5% per year in the comparator group. The overall estimated number-needed-to-harm for congestive heart failure was 107 over the 29•7-month mean follow-up. Table 2 shows that the number-needed-to-harm varied across studies from 35 in one trial10 to 491 in another.19
Sequential exclusion of each trial from the analysis of congestive heart failure and cardiovascular death did not affect the overall relative risks. To account for heterogeneity in the studied populations, we excluded trials of patients with prediabetes and with metabolic syndrome without type 2 diabetes;18,21 this did not affect the RR for congestive heart failure (1•46, 1•19-1•78, p=0•0003) or cardiovascular death (0•91, 0•63-1•30, p=0•59). Neither did exclusion of a trial that enrolled a diabetic population with established and treated heart failure10 affect the RR for congestive heart failure (1•74, 1•21-2•50, p=0•003) or that for cardiovascular death (0•91, 0•65-1•28, p=0•60). The overall rate of congestive heart failure was 1•8% (360 of 20 191 patients). If only trials with placebo-control groups were included, the RR for congestive heart failure was 1•97 (95% CI 0•94-4•13, p=0•07), and the RR for cardiovascular death was 1•08 (0•66-1•76, p=0•77).
Pooled results from each of the TZD trials showed an increased risk for congestive heart failure was associated with both rosiglitazone and pioglitazone (table 3, figure 2). The pooled RR for development of congestive heart failure was 2•18 (95% CI 1•44-3•32, p=0•0003) in the five trials of rosiglitazone, and 1•32 (1•04-1•68, p=0•02) in two studies with pioglitazone. Figure 3 shows that the pooled RR for cardiovascular death was not higher than in controls with either rosiglitazone (RR 0•91, 95% CI 0•63-1•32, p=0•63) or pioglitazone (1•01, 0•51-2•01, p=0•98).
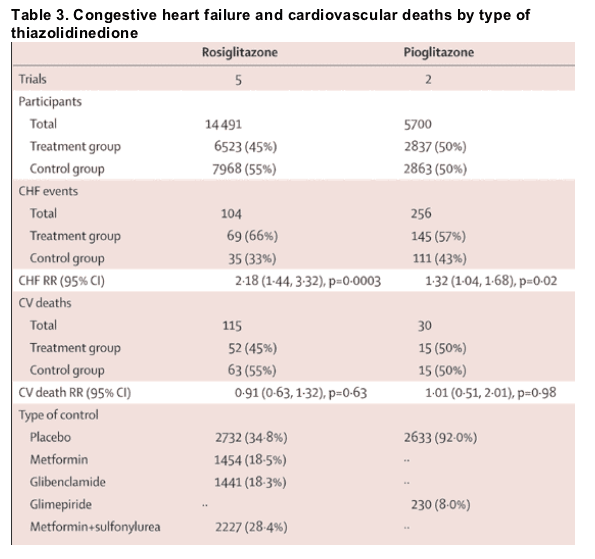
Table 2 summarises the raw number of events in the trials we assessed and the number of events per year. The risk for congestive heart failure did not differ for rosiglitazone and pioglitazone (1•74, 0•97-3•14, p=0•07). The risk of cardiovascular death did not differ between both drug groups (1•01, 0•73-1•40, p=0•96) (figure 4). The funnel plots were asymmetric, which suggested a publication bias, but this was not sufficiently large to affect our results or interpretations in a meaningful way.
Figure 4. Comparison of risk of congestive heart failure (A) and cardiovascular death (B) for rosiglitazone and pioglitazone
TZDs=thiazolidinediones. Risk ratios (RR) are shown on a logarithmic scale. The area of each square is proportional to the weight of the individual study, measured as the inverse of the estimated variance of the log risk ratio. The diamond represents the pooled relative risk and its width represents its 95% CI. df=degrees of freedom.
Discussion
In 20 191 patients with prediabetes or type 2 diabetes in seven randomised trials, the risk of congestive heart failure was higher in patients given TZDs than in controls. However, despite the higher incidence of congestive heart failure in patients given TZDs, these patients did not have a higher rate of cardiovascular death. Even with the expected heterogeneity of baseline risk for congestive heart failure in these patients, the final outcome, measured for either congestive heart failure or cardiovascular death, was not affected by exclusion of any specific trial or group of trials in which patients' characteristics were different.
The relative risk for congestive heart failure was increased across a wide background of cardiovascular risk in these trials: patients with prediabetes;18,21 those with type 2 diabetes without cardiovascular disease;19,20 with type 2 diabetes and established cardiovascular disease without congestive heart failure;7 and with type 2 diabetes with documented congestive heart failure (NYHA class I and II) and an ejection fraction of less than 40%.10 However, although the relative risk for congestive heart failure was similar across the trials, the absolute risk varied according to the severity of the glucometabolic state and the presence or absence and degree of cardiovascular disease at baseline. Except in one trial,10 congestive heart failure occurred in the absence of any history or clinical evidence of congestive heart failure or systolic dysfunction at the time of enrolment.
Since drug exposures in these trials were short, and patients did not have previous histories of congestive heart failure or evidence of left ventricular dysfunction at entry, the excess of congestive heart failure events related to TZDs was probably the result of TZD-related fluid retention and diastolic dysfunction in susceptible patients. The natural history of congestive heart failure when caused by TZD-related fluid retention is unknown. A recent study23 suggested that either amiloride or spironolactone could induce diuresis in rosiglitazone-induced fluid retention; this effect is consistent with activity of the PPARϒ agonists in the distal collecting duct.24 Several factors can affect left ventricular diastolic function in type 2 diabetes25-32 such that a small increase in plasma volume (as noted in patients given TZDs) could precipitate pulmonary oedema because of an increase in left atrial and pulmonary venous pressures.33-35
Diabetes is regarded to be commonly associated with diastolic dysfunction.30,31 Devereux and colleagues35 showed that type 2 diabetes might be associated with an increased prevalence of diastolic dysfunction, especially in those with hypertension. In most studies, patients who have an episode of heart failure due to diastolic dysfunction have a similarly poor prognosis to patients with systolic heart failure, at least in older patients with comorbidities.32,33,36-44 However, these epidemiological studies have not addressed whether an episode of heart failure that might be unmasked by fluid retention would have a different outcome when compared with the occurrence of diastolic heart failure in the general population.
This meta-analysis has shown that the increased event rate of congestive heart failure with TZDs was not associated with an increased risk of cardiovascular death in these trials. One observational study45 reported that, ambulatory patients with congestive heart failure given TZDs did not have a higher risk of admission to hospital for congestive heart failure or death than those who did not receive TZDs. Another observational study showed that, when compared with other antidiabetic drugs, insulin-sensitisers did not change the risk of death within a year after acute myocardial infarction, although the risk of readmission to hospital for heart failure was higher.46
A meta-analysis of three randomised clinical trials (one with prediabetic patients and two with type 2 diabetic patients with a history of either cardiovascular disease or congestive heart failure) and four observational studies showed that the risk of congestive heart failure doubled with use of TZDs.47 Our meta-analysis extended this by including four additional randomised trials,8,19-21 with patients across a wider background of cardiac risk, that compared TZDs both with placebo and with active treatments. Our meta-analysis also assessed cardiovascular death in these trials.
A recent meta-analysis showed that patients given rosiglitazone had a higher risk of myocardial infarction than controls; they also had a higher risk of cardiovascular death, although this was not significant.9 By contrast, our meta-analysis did not show an increased hazard of cardiovascular death for either rosiglitazone or pioglitazone, despite the increased risk of congestive heart failure, even though the occurrence of congestive heart failure confers a poor prognosis for patients with type 2 diabetes.26,30 We did not include the smaller trials48 available for either rosiglitazone or pioglitazone, since they might not have had long enough observation times to accurately measure the risk for congestive heart failure and cardiovascular death. Furthermore, in these smaller studies, congestive heart failure events were defined in variable terms, investigator-reported, and not adjudicated.
Whether TZD-related fluid retention is a more benign cause of congestive heart failure than other causes cannot be confirmed without a comparison of outcomes between patients who developed congestive heart failure in the treatment and control groups. In one trial,7 mortality due to heart failure was similar in both the pioglitazone and placebo groups. In a more recent analysis of this trial, more cases of serious heart failure were associated with pioglitazone than with placebo, but the number of primary and main secondary events were similar in each group.49 One interpretation of these findings could be that congestive heart failure in the pioglitazone group (which was probably the result of fluid retention in many patients) carried a more favourable overall prognosis than did congestive heart failure in the placebo group (which was caused by progressive cardiac dysfunction). Another explanation could be that despite the potential for more adverse cardiovascular events associated with congestive heart failure, pioglitazone could have a cardioprotective effect compared with placebo.
We did not have data about the outcomes of patients in whom congestive heart failure was due to fluid retention, to enable comparison with those in whom it was due to other causes. Moreover, the overall benefit for patients given pioglitazone in the main secondary endpoint compared with controls, could be explained by pioglitazone-related directional changes in blood pressure, high-density lipoproteins, triglycerides, and glycaemia, which are factors associated with a lower cardiac risk. Any intrinsic negative effect of pioglitazone related to the risk of congestive heart failure or coronary ischaemia could have been counterbalanced or negated by beneficial changes in these cardiovascular risk factors. The true risk-benefit profile of a TZD when compared with another treatment for diabetes should be assessed when glycaemia and other cardiovascular risk factors are similar in the two treatment groups. Despite the glucose-lowering effect of TZDs, our data indicate that these drugs should not be used in patients with heart failure and should be cautiously used for glycaemic control in patients with cardiovascular disease who do not have heart failure. In patients with type 2 diabetes without cardiovascular disease in whom the absolute risk for congestive heart failure is much lower, the use of TZDs should be weighed against the risks and benefits of other antidiabetic medications.
Limitations of this study included the inherent assumptions made for any meta-analysis, and the use of aggregated data either as reported or as provided by individual study authors. Individual patient data and original data were not available, which limited our ability to do more detailed time-to-event analyses. The meta-analytic approach used might not have detected methodological problems in the primary studies. Moreover, definitions of heart failure differed between the included trials. Another potential limitation was that the comparator populations included both placebo and active treatments. The incidence of heart failure across trials was also heterogeneous across trials.7,18 Moreover, information about patient-specific data did not include the outcomes of congestive heart failure in the TZD and the control groups. Insufficient follow-up durations could have affected our conclusions about the association between congestive heart failure and cardiovascular mortality. We also did not have sufficient data to assess whether the risk of congestive heart failure differed between the two TZDs. We need longer follow-up and better characterisation of patients in whom congestive heart failure develops because of fluid retention to determine the effect of TZDs on overall cardiovascular outcome and whether congestive heart failure should be regarded as an adverse event or a characteristic cardiovascular endpoint.
Methods
Search strategy
Randomised, double-blind, controlled trials of TZDs were eligible for inclusion in our meta-analysis if they reported risk estimates or frequency data for congestive heart failure and cardiovascular death. We excluded non-randomised clinical trials and those in which outcomes were not reported.
We did a systematic review of Embase, MEDLINE, Database of Abstracts of Reviews of Effects (DARE), and the Cochrane Library (from January, 1998, to March, 2007). Figure 1 summarises the flowchart of article selection and inclusion. We searched for the following MeSH terms: "heart failure, congestive", "mortality", "cardiovascular system", OR "edema" OR the text words "cardiovascular", "CHF", "edema", "cardiac", "heart", "death", "mortality", OR "congestive heart failure", OR "adverse" [all fields] AND "events" [all fields] AND (the MeSH term "thiazolidinediones" OR the text word "thiazolidinediones") OR "TZD" [all fields] OR ("rosiglitazone" [substance name] OR "rosiglitazone" [text word]) OR ("pioglitazone" [substance name] OR "pioglitazone" [text word]). We selected only randomised controlled trials with male human patients that were written in English. We also searched the databases of the European Society of Cardiology, American Heart Association, American College of Cardiology, and American Diabetes Association by hand to identify full publications that had not yet been indexed. We reviewed bibliographies of retrieved publications to further increase the yield of potentially relevant articles.
Data collection and quality assessment
Two independent investigators extracted and tabulated data in a standardised data-extraction form. Discrepancies were resolved by group discussions and by reference to the original reports. The standard form included first author, publication year, mean age of participants, sex proportion, trial duration, type of TZD agent, type of control or controls, number of participants in drug and control groups, and number of events of interest (congestive heart failure and cardiovascular death) in the drug and control groups.
We measured the quality of these trials on the basis of internal validity and control of selection bias, detection bias, and attrition bias. We assessed selection bias according to incorporation of age, gender, and cardiac-disease history in risk estimates, when applicable. For control of attrition bias, we assessed the extent of loss to follow-up, represented as a proportion of the total initial study population. We calculated the loss-event ratio, from the number of patients lost to follow-up and the number of outcome events in the study, to assess the importance of loss to follow-up risk estimates in each study. We arbitrarily regarded studies with loss-event ratios of less than 10% as having satisfactory control of attrition bias.
Statistical analysis
We allocated the results of each randomised control trial as dichotomous frequency data. We did separate meta-analyses with the DerSimonian and Laird13 random-effects models to obtain pooled relative risks (risk ratios, RR) and associated 95% CIs for outcomes, with an intention-to-treat approach as a measure of association. Natural log transformations were done on the RR calculations. All analyses were initially done with a fixed-effects model, and were repeated if we detected heterogeneity across studies with a random-effects model, which included a measure of variance in the calculation of pooled results. Conventional random-effects weighting was used in all analyses. All p values were two-sided and p values of less than 0•05 were regarded as significant. We also did subgroup analyses with meta-regression to assess potential effect modification by type of control. To avoid statistical duplication of data, multiple control groups in a trial were collapsed as one independent control group for comparison to a specific TZD agent.
For studies that reported no events in a trial group, we applied Haldane's correction with the classic half-integer correction to calculate the RRs and variances. We did further sensitivity analyses of data with Mantel-Haenszel weighted pooling of trials. We used the I2 test to assess the percentage of total variation across studies that was due to heterogeneity rather than chance; to quantify inconsistency across studies; and to check that results from individual studies could be pooled.14,15
To check for potential publication bias we used both Egger's test and Begg's tests.16 Accordingly, we examined for relative symmetry of individual study estimates around the overall estimate with Begg funnel plots in which log RRs were plotted against their corresponding standard errors. All primary analyses were done with Review Manager statistical software (version 5.0; The Cochrane Collaboration, Oxford, England; 2007), and additional analyses with Comprehensive Meta Analysis (version 2, Biostat, Englewood, NJ, USA).17
Role of funding source
No funding source was involved in the conception or development of the study. The first author and corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
|
|
| |
| |
|
|
|