 |
 |
 |
| |
Efficacy and Safety of Atazanavir-Based Therapy in Antiretroviral-Naive HIV-1-Infected Subjects,
Both With and Without Ritonavir: 96-Week Results From AI424-089
|
| |
| |
Reported by Jules Levin
4th IAS Conference, 22-25 July, Sydney, Australia
N. Malan1, E. Krantz2, N. David3, M. Mathew4, A. Rightmire4, V. Wirtz4, R. Yang4, and D. McGrath4 for the BMS AI424-089 Study Group
1Triple M Research, Port Elizabeth, South Africa; 2Quinta-Research, Bloemfontein, South Africa; 3Brooklyn Medical Centre, Cape Town, South Africa; and 4Research and Development, Bristol-Myers Squibb, Wallingford, Connecticut, United States
AUTHOR DISCUSSION
There was no significant difference in efficacy between ATV300/RTV- and ATV400-based regimens with both regimens being effective in achieving and maintaining virologic response over 96 weeks in ARV-naive subjects with HIV infection. Robust CD4 cell count increases were observed with both regimens
While not statistically significant, rates of response (TLOVR) were higher for HIV RNA <400 copies/mL, and even more so for <50 copies/mL, in subjects on ATV300/RTV
There were fewer virologic failures among subjects on ATV300/RTV
PI and NRTI substitutions occurred more frequently in subjects experiencing virologic failure on the ATV400-based regimen
No subject with virologic failure on ATV300/RTV developed phenotypic resistance to ATV or any other PI. In contrast, 4 subjects with virologic failure on ATV400 developed phenotypic resistance to ATV, and one developed phenotypic resistance to nelfinavir
The increase in lipids seen on both regimens is consistent with a known return to health effect observed in treatment-naive subjects started on HAART. The increases in triglycerides are consistent with the known impact of prolonged exposure to stavudine and were lower in both arms than have been observed in other studies of stavudine-containing HAART.5 The majority of subjects on both regimens remained within the NCEP categories 1 and 2, where they were
at baseline
No subjects in the ATV300/RTV arm discontinued therapy because of an AE after week 48, although this arm was associated with higher rates of hyperbilirubinemia through week 96 compared with ATV400. There were few AE-related discontinuations in either arm, suggesting that AEs were generally manageable
AUTHORS CONCLUDED
ATV, with or without RTV, given once daily as part of a HAART regimen,
demonstrated a high rate of virologic response through 96 weeks in
treatment-naive subjects, including those with advanced HIV disease.
Both arms were generally safe and well-tolerated. These results support
additional studies of ATV300/RTV in ARV-naive subjects
INTRODUCTION
Atazanavir (ATV) is a potent, generally well-tolerated, once-daily protease inhibitor (PI) that is recommended for the treatment of HIV infection in treatment-naive patients based on efficacy comparable to that of efavirenz1,2
The efficacy and safety of ritonavir (RTV)-boosted ATV have been demonstrated in antiretroviral (ARV)-experienced patients over 96 weeks.3 However, long-term data on its use in treatment-naive patients are lacking
The AI424-089 study was a randomized, open-label, multicenter, 96-week study designed to compare the efficacy and safety of ATV/RTV to ATV, each given once daily in combination with once-daily 3TC and extended-release stavudine (d4T; not commercially available) in HIV treatment-naive patients
The 48-week results of this trial have been described previously.4 Here, we present the results of the planned 96-week efficacy and safety analyses
METHODS
In the AI424-089 study, eligibility criteria included HIV-1 infection with a plasma HIV RNA level >2000 copies/mL, any CD4 cell count, and that patients were ARV-naive
Patients were randomized (1:1) to:
-- ATV 300 mg + RTV 100 mg + 3TC 300 mg + d4T extended-release 100 mg, all
administered together once daily (ATV300/RTV)
--ATV 400 mg + 3TC 300 mg + d4T extended-release 100 mg, all administered together once daily (ATV400)
Virologic efficacy was assessed using both ITT (time to loss of virologic response [TLOVR]) and on-treatment methods of analysis
ATV300/RTV would be considered non-inferior to ATV400 if the lower limit of the 95% confidence interval (CI) for the difference in virologic efficacy (TLOVR analysis) between the two treatment arms (ATV300/RTV and ATV400) was greater than -10%
Other parameters assessed were CD4 cell count, adverse events (AEs), laboratory abnormalities, and plasma lipid levels
RESULTS
200 patients were randomized, and all but one were treated
The treatment arms were balanced with regard to demographics and HIV disease characteristics (Table 1). Subject disposition at 96 weeks is shown in Table 2
Efficacy
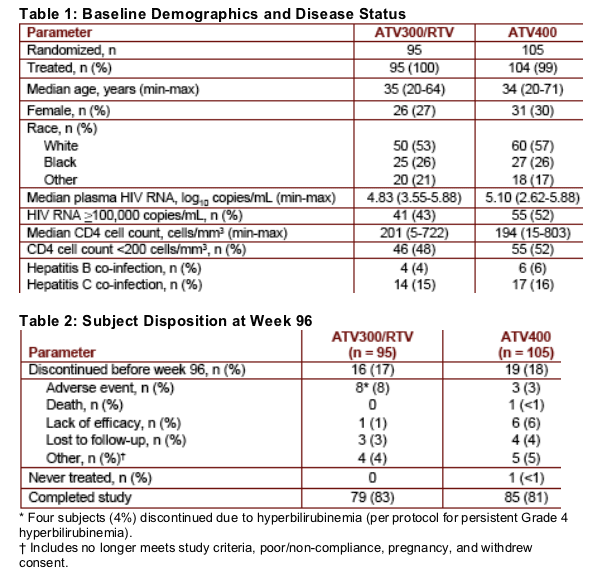
ATV300/RTV was non-inferior to ATV400 at both limits of detection (400 copies/mL and 50 copies/mL) in the TLOVR analyses through week 96 (Figure 1)
Figure 1: Percentage of Subjects Achieving HIV RNA Levels <400 copies/mL and <50 copies/mL: TLOVR Analysis
<400: 75% ETV/r, 70% ATV (difference estimate: 5.1 [-7.1, 17.2])
<50: 65% ATV/r, 55% ATV (difference estimate: 9.8 [-3.4, 23.1])
Response rates were slightly higher among the ATV300/RTV than ATV400 recipients in the on-treatment analyses: at 96 weeks, 94% (74/79) and 87% (74/85) had HIV RNA levels <400 copies/mL, and 84% (66/79) and 76% (65/85) had HIV levels <50 copies/mL,
respectively
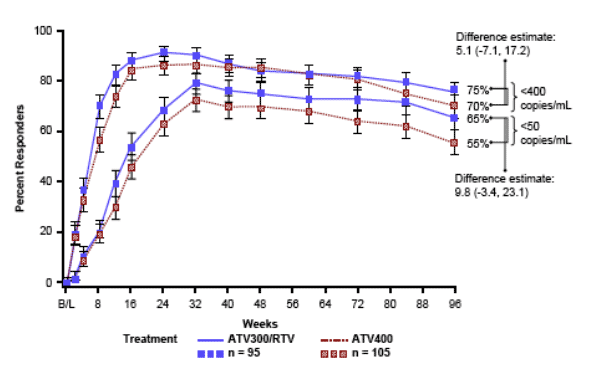
Subjects on either ATV300/RTV or ATV400 demonstrated rapid and potent suppression of plasma HIV RNA levels that was sustained throughout the 96-week study period (Figure 2)
Mean changes in HIV RNA levels (baseline to 96 weeks) were -3.18 and
-3.09 log10 copies/mL for the ATV300/RTV and ATV400 regimens, respectively
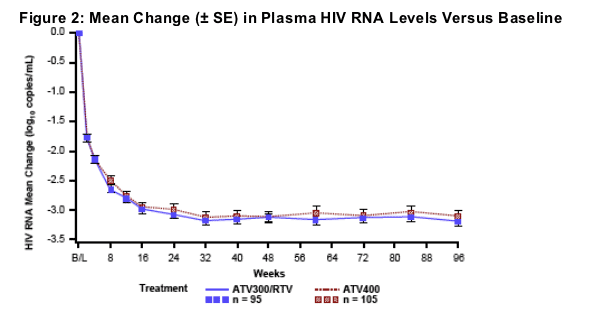
Mean changes in CD4 cell count from baseline to week 96 were +276 and +315 cells/mm3 for the ATV300/RTV and ATV400 regimens, respectively (Figure 3)
Five subjects (5%) experienced virologic failure (all virologic rebounds: confirmed HIV RNA >400 copies/mL) following an initial response to ATV300/RTV, compared with 20 subjects (16 [15%] virologic rebounds and 4 discontinued due to insufficient viral load response) in the ATV400 arm. Only 2 subjects on ATV300/RTV experienced rebound between weeks 48 and 96, compared with 10 subjects in the ATV400 arm
Resistance
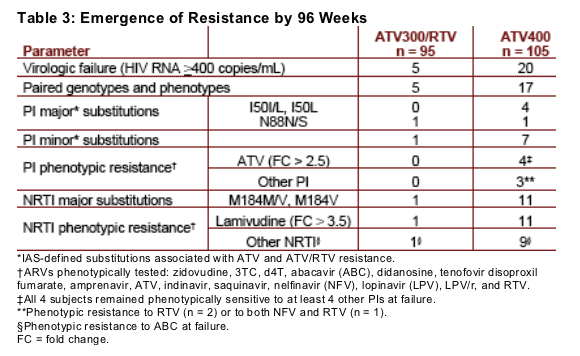
Treatment-emergent drug resistance is summarized in Table 3
Virologic failure occurred in 5 subjects on ATV300/RTV and in 20 subjects on ATV400
Paired baseline and on-treatment genotypic data were available for all 5 subjects with virologic failure on ATV300/RTV, and 17 of 20 subjects with virologic failure on ATV400
No subjects with virologic failure in the ATV300/RTV arm developed on-treatment phenotypic resistance to ATV, compared with 4 subjects in the ATV400 arm (fold changes [FC]: 2.58, 5.85, 11.0, and 26.0)
Treatment-emergent major (IAS-defined) protease substitutions associated with phenotypic resistance to ATV were I50L (n = 1; FC = 11) and I50L+N88N/S (n = 1; FC = 26). The other 2 subjects with phenotypic resistance to ATV at virologic failure did not have major protease substitutions as defined by IAS
Safety
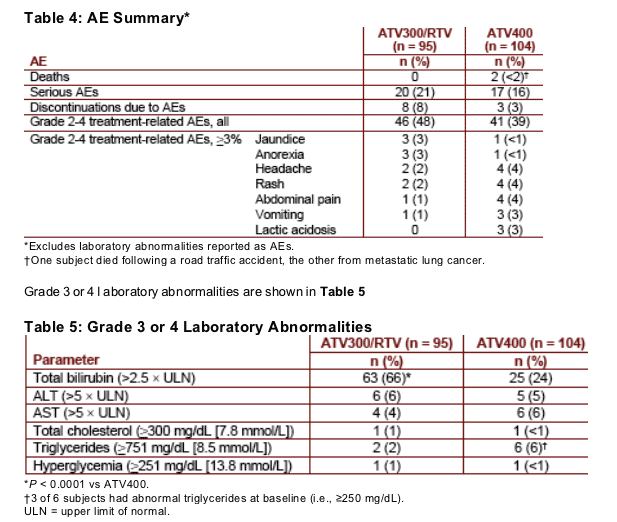
Safety data are summarized in Table 4
Eight subjects discontinued ATV300/RTV because of AEs, all during the first 48 weeks of treatment. AEs leading to discontinuation included hyperbilirubinemia (3 subjects), hyperbilirubinemia and jaundice (1 subject), blood bilirubin abnormalities (1 subject), rash
(1 subject), bipolar disorder (1 subject), and vomiting (1 subject)
More than half of all subjects in the ATV300/RTV arm who discontinued by week 48 due to AEs were required to do so by protocol mandate, as a result of persistent grade 4 hyperbilirubinemia, regardless of clinical symptoms
Grade 3 or 4 treatment-related AEs occurred in 28% and 19% of subjects in the ATV300/RTV and ATV400 arms, respectively
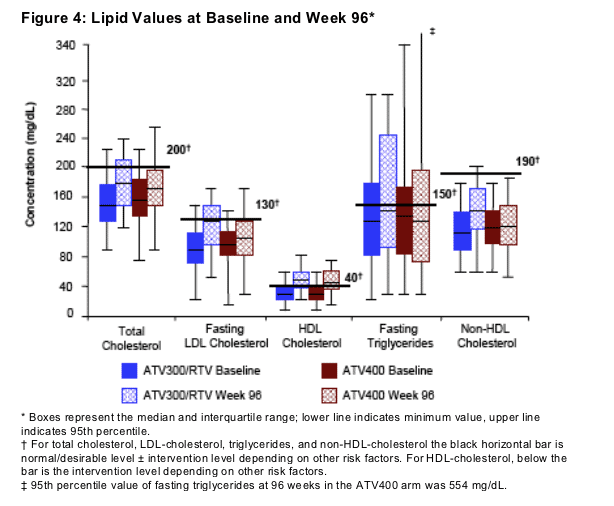
Changes in plasma lipids are shown in Figure 4
The majority of the changes in each lipid parameter were generally seen during the first 48 weeks of treatment, with less change occurring thereafter
Median levels of HDL-cholesterol increased by 33% and 23% in the ATV300/RTV and ATV400 arms, respectively, between baseline and 96 weeks
Median increases in total cholesterol were 20% and 7%, and in LDL-cholesterol were 27% and 14% in the ATV300/RTV and ATV400 arms, respectively, between baseline and 96 weeks
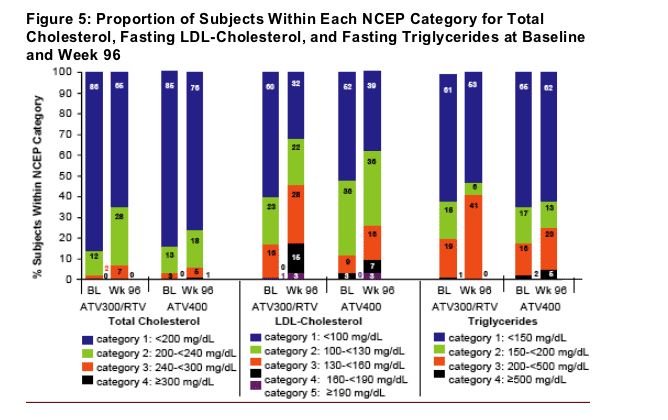
Proportion of subjects in each National Cholesterol Education Program (NCEP) category for total cholesterol, fasting LDL-cholesterol, and fasting triglycerides at baseline and at 96 weeks
is shown in Figure 5
REFERENCES
1. Hammer SM et al. JAMA. 2006;296:827-843.
2. Squires K et al. J Acquir Immune Defic Syndr. 2004;36:1011-1019.
3. Johnson M et al. AIDS. 2006;20:711-718.
4. Malan N et al. Abstract presented at: 13th CROI; Denver, Colorado; February 5-8, 2006. Abstract 107LB.
5. Gallant JE et al. JAMA. 2004;292:191-201.
|
| |
|
 |
 |
|
|