 |
 |
 |
| |
Likely Resistance to Etravirine Possibly Under 2% in French Cohort
|
| |
| |
4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention
July 22-25, 2007
Sydney, Australia
Mark Mascolini
Only 1.2% of a French HIV population carried virus that may prove resistant to etravirine (TMC125), the second-generation nonnucleoside (NNRTI) now in late-stage development [1]. The results back up findings in a similar Spanish study [2] but must be interpreted cautiously because which mutations or mutation sets make HIV resistant to etravirine requires closer study.
The French team analyzed viral genotypes from 1686 people for two sets of mutations: (1) an NNRTI set of mutations that cause resistance to efavirenz, nevirapine, or both, culled from the Stanford University database, the ANRS mutation set, the IAS-USA mutation set, and a Tibotec mutation set, and (2) an etravirine-related set of mutations based on (a) Tibotec in vitro resistance studies and (b) the phase 3 DUET trials [3]. The viral samples analyzed came primarily from people infected with HIV-1 subtype B (67%) or AG (18%).
Because a mutation algorithm predicting resistance to etravirine has not been proposed, the French investigators devised one of their own. They considered resistance to etravirine likely when HIV had one of two patterns:
1. Two or more NNRTI mutation including at least one mutation from the Tibotec resistance studies
2. Three or more mutations from the phase 3 DUET studies (V90I, A98G, L100I, K101E, K101P, V106I, V179D, V179F, Y181C, Y181I, Y181V, G190A, and G190S) [3]
The French study did not consider Y318F, which is counted as an etravirine mutation in the Stanford database and the in vitro Tibotec set, because it lay outside the reverse transcriptase region in which they searched for mutations.
More than one quarter of the analyzed sequences (28.8%) had at least one NNRTI mutation from the Stanford database, while 22.1% had one or more IAS-USA nonnuke mutations, and 22.4% had one or more ANRS nonnucleoside mutations (Table). According to the ANRS resistance algorithm, this means 22.4% of the viral samples tested would have high-level resistance to efavirenz, nevirapine, or both.
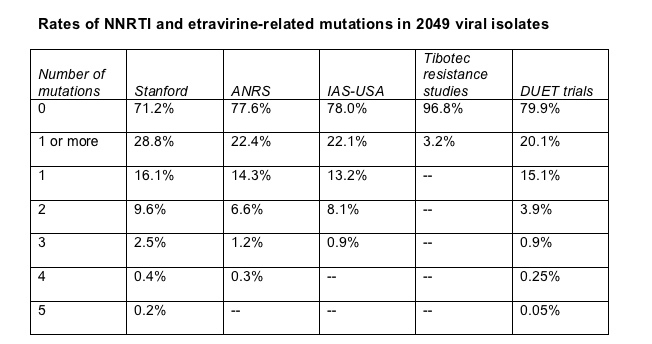
Individual etravirine-related mutations described in the DUET trials were not scarce in this cohort, turning up in 15.1% of the Aquitaine samples. And 20.1% of samples had one or more such mutations (Table). But only 1.2% of viral sequences carried three or more etravirine-related mutations defined in the DUET trials and were thus considered potentially resistant to etravirine according to the definitions established for this Aquitaine study. The most frequent DUET mutations in the Aquitaine cohort were Y181C in 6.1%, G190A in 4.4%, V90I in 3.5%, and V106I in 3%. Only 3.2% of samples had one or more of the etravirine mutations seen in Tibotec's resistance studies.
A single viral sequence analyzed had three or more etravirine-linked mutations identified in DUET and no ANRS or IAS-USA mutations. Of all the sequences scrutinized, this was the only one possibly resistant to etravirine and susceptible to efavirenz and nevirapine.
The Aquitaine team concluded that mutation patterns potentially conferring resistance to etravirine are rare in clinical practice. The investigators urged judicious use of efavirenz or nevirapine to prevent emergence of mutations that may render HIV resistant to etravirine.
The precision of this analysis remains open to question because resistance to etravirine requires much more study. For example, the Aquitaine definitions of resistance to etravirine changed substantially between the time these researchers submitted their abstract for this conference and the time they presented their poster. Still, the French results and those of the 1586-person Spanish study [2] both suggest that few people in Western Europe carry mutations that may readily make their virus resistant to etravirine. And in the DUET trials, 86% of study participants had two or fewer etravirine-related mutations [3]. Of course the antiviral impact of etravirine in people with virus already resistant to efavirenz or nevirapine will also depend on which other antiretrovirals they can combine with this new NNRTI in a rescue regimen.
References
1. Cotte L, Trabaud MA, Tardy JC, et al. HIV-1 NNRTI mutation profiles in clinical practice: implications for TMC125 use: 4th IAS Conference on HIV Pathogenesis, Treatment, and Prevention. July 22-25, 2007. Sydney. Abstract TUPEB033.
2. Llibre JM, Santos JR, Puig T, et al. Prevalence of etravirine-associated mutations in
clinical samples with resistance to nevirapine and efavirenz. Antiviral Therapy. 2007;12:S74. Abstract 65.
3. Vingerhoets J, Buelens A, Peeters M, et al. Impact of baseline NNRTI mutations on the virological response to TMC125 in the phase III clinical trials DUET-1 and DUET-2. Antiviral Therapy. 2007;12:S34. Abstract 32.
|
| |
|
 |
 |
|
|