 |
 |
 |
| |
Efficacy and safety of NRTI's switch to tenofovir plus emtricitabine (Truvada) vs. abacavir plus lamivudine (Kivexa) in patients with virologic suppression receiving a lamivudine containing HAART: The BICOMBO study
|
| |
| |
Reported by Jules Levin
4th IAS Conference, 22-25 July, Sydney, Australia
Martinez E.1, Arranz J.A.2, Podzamczer D.3, Ribera E.4, Knobel H.5, Roca V. 6, Gutierrez F.7, Llibre J.M.8, Sans J. 2, Barragan P.3, Clotet B.9, Dalmau D.10, Segura F.13, Arribas J.R.14, Cosin J15., Force L.16, Palleras A.17, de los Santos I.18, Peraire J.19, Galo F.20, Pich J.11, de Lazzari E.12, Gatell J.M.1 , for the BICOMBO study group
Tenofovir (TDF) plus emtricitabine (Truvada) and abacavir (ABC) plus lamivudine (Kivexa) are fixed dose combinations and the most commonly recommended and used nucleoside analogs backbone for initial antiretroviral therapy. No head to head comparison has been performed so far between Truvada and Kivexa.
AUTHOR CONCLUSIONS
In patients switching from a lamivudine containing regimen:
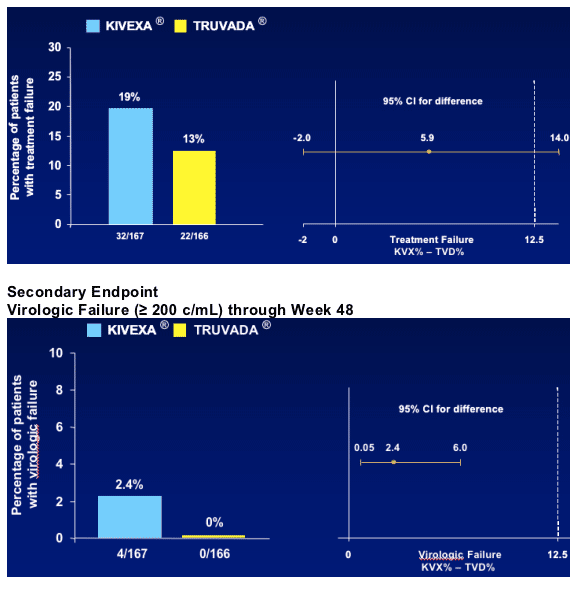
For treatment efficacy, the Kivexa group did not meet the non-inferiority endpoint compared to the Truvada group
--the difference was mainly driven by Kivexa discontinuations due to suspected abacavir hypersensitivity
For the virologic efficacy, Kivexa met non-inferiority criteria compared to Truvada
--however, there were more failures with Kivexa than Truvada (2.4% vs 0%)
On switching from a lamivudine nucleoside backbone to either Kivexa or Truvada :
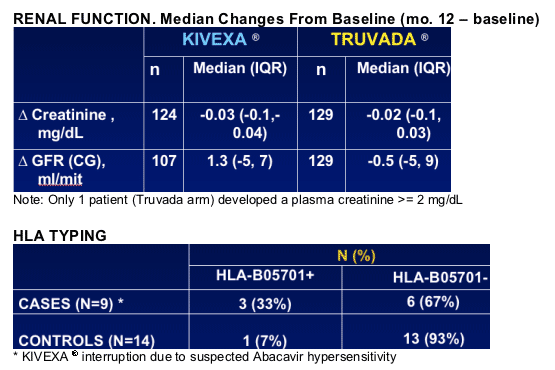
--Retrospective HLA testing showed B5701+ in 3/9 cases of suspected HSR
--Safety endpoints showed:
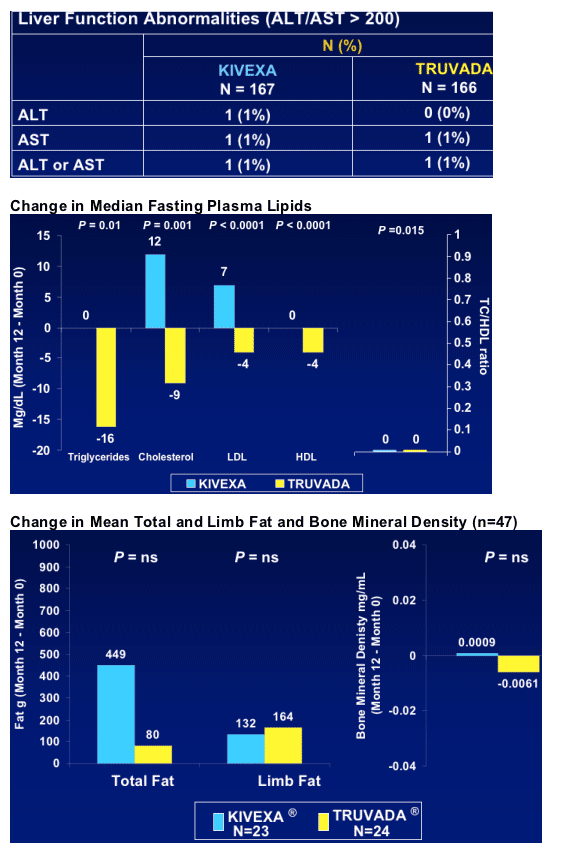
A more favorable lipid profile (cholesterol, TG and LDL but not HDL) for those switching to Truvada vs Kivexa
No differences in renal function or bone mineral density between Truvada and Kivexa treatment arms
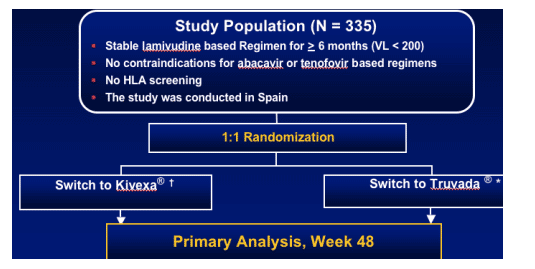
OBJECTIVE
To compare the efficacy and safety of Truvada vs. Kivexa in patients with virologic suppression and receiving a lamivudine containing regimen.
Study Design
Tenofovir 300 mg plus emtricitabine 200 mg + unchanged NNRTI or PI.
Abacavir 600 mg plus lamivudine 300 mg + unchanged NNRTI or PI.
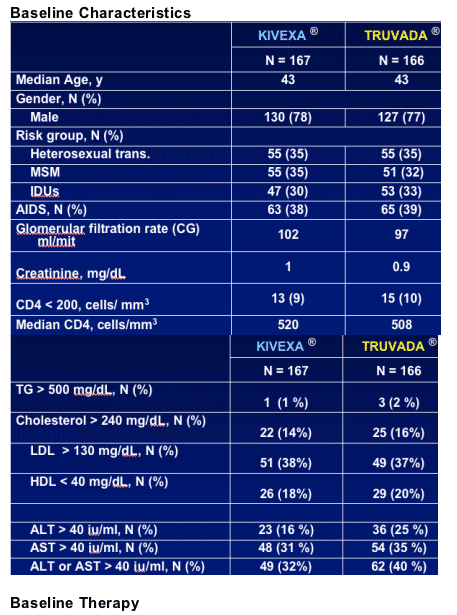
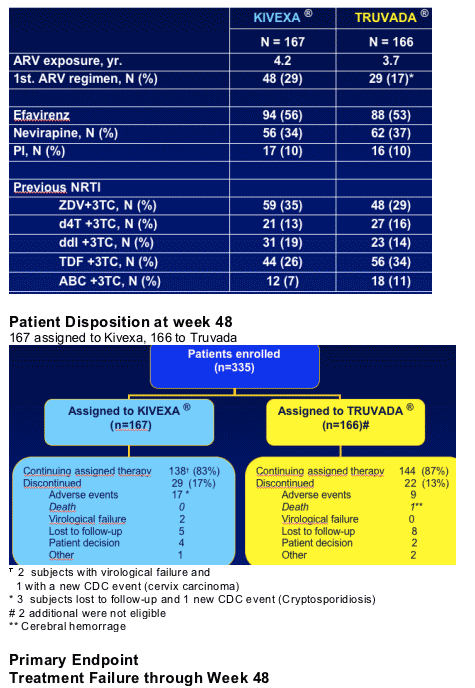
335 patients on stable 3TC based regimen for >6 months with <200 c/ml; no HLS screening; study conducted in Spain. Patients were switched to Kivexa or Truvada. Primary analysis, Week 48.
Primary Endpoint
The proportion of patients with treatment failure for any reason through Week 48
--Includes virologic rebound (> 200 cp/mL), discontinuation of study therapy or lost to follow-up, progression to a new CDC category C event or death.
--Non-inferiority study of Kivexa vs Truvada. Upper limit of 95% CI of estimated difference < 12.5%.
Secondary Endpoints
The proportion of patients with virologic failure at or prior to week 48
--Confirmed on-study HIV RNA ³ 200 copies/mL or last on-study HIV RNA ³ 200 copies/mL followed by discontinuation
Time to treatment failure and to virologic failure
CD4 changes
Safety
--Changes of fasting plasma lipids, body fat, bone mineral density and renal function
4 patients developed VF in the Kivexa arm
4 / 4 no resistance tests before any ART regimen
4 / 4 two or more previous ART regimens for 1-5 years. Never exposed to ABC
1 / 4 previous virologic failure. Wild type virus
VF developed between months 4-8 of the study
4 / 4 were receiving Kivexa + Nevirapine or Efavirenz
2 / 4 genotypic test available at failure
75I, 184V, 219E, 101E, 181E
74V, 100I, 103 ,63S
4 / 4 VL became undetectable with same (n=1) or different ART (n=3)
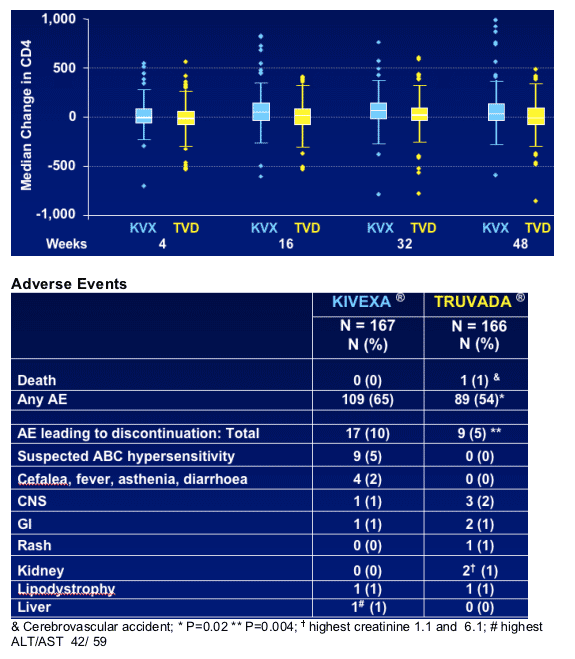
CD4 Changes
Median changes in CD4 cell count were: +44 cells/mm3 (KIVEXA ) and - 2.7 cells/mm3 (TRUVADA ) through Week 48 (p=0.032)
LAB Abnormalities (liver abnormalities)
|
| |
|
 |
 |
|
|