 |
 |
 |
| |
ABC107442 (SHAPE)
Study of Hypersensitivity to Abacavir and Pharmacogenetic Evaluation
|
| |
| |
Reported by Jules Levin
4th IAS, 22-25 July, Sydney, Australia
Michael Saag, Rukmini Balu, Elizabeth Phillips,* Philip Brachman, Claudia Martorell, William Burman, Britt Stancil, Michael Mosteller, Cindy Brothers, Paul Wannamaker, Arlene Hughes, Denise Sutherland-Phillips, Simon Mallal and Mark Shaefer for the SHAPE team
AUTHOR CONCLUSIONS:
Results in Whites consistent with the randomized study PREDICT-1 (Abstract WESS101) that demonstrated pre-therapy screening for HLA-B*5701 significantly reduced both clinical and immunologically confirmed ABC HSR.
Similar results seen in Blacks imply that screening for HLA-B*5701 is a broadly generalizable approach for reducing ABC HSR rates.
Background
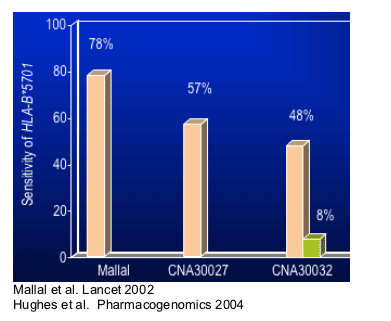
Studies have observed variable sensitivity of HLA-B*5701
--Definition of ABC HSR and clinical phenotyping
false positive clinical diagnosis
--Differences in white and nonwhite races
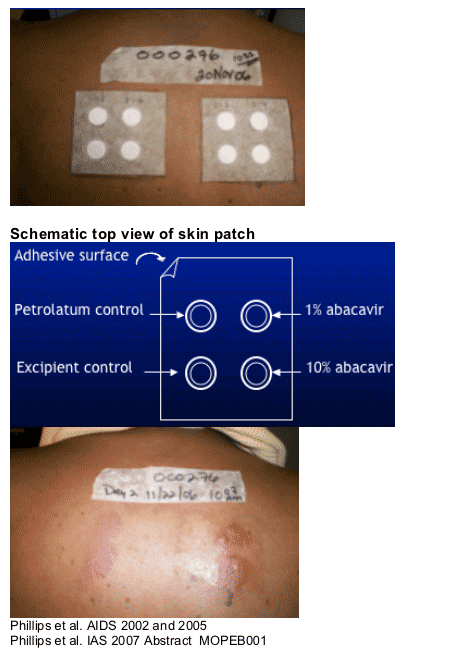
Abacavir Skin Patch Testing
--Immune cell-mediated reaction
-- Research tool used to identify patients with immune-mediated abacavir HSR
What is SHAPE?
Retrospective case-control study
--To evaluate the sensitivity and specificity of HLA-B*5701
--Study design necessary because of low carriage frequency of HLA-B*5701 and low rates of ABC HSR in Blacks
Addresses two important aspects of pharmacogenetic research
--Generalizability to White and Black races
--Supplement clinical definition of ABC HSR with patch test (phenotype)
SHAPE is a retrospective study. Subjects must have already taken abacavir in order to be considered either a case or a control.

Overall study design of SHAPE - cases on the left and controls on the right. This study did not enroll controls. Rather controls were identified from existing clinical study databases and were not required to undergo any new procedures. Only cases underwent skin patch testing.
RESULTS
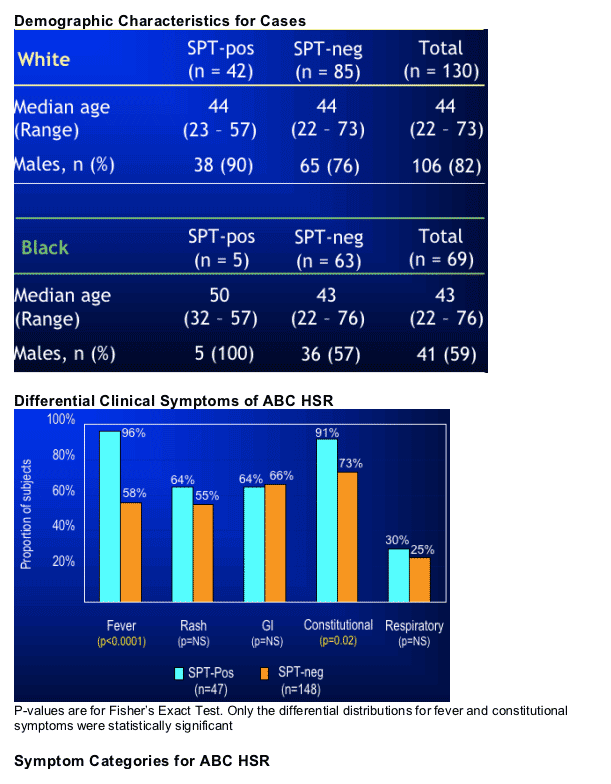
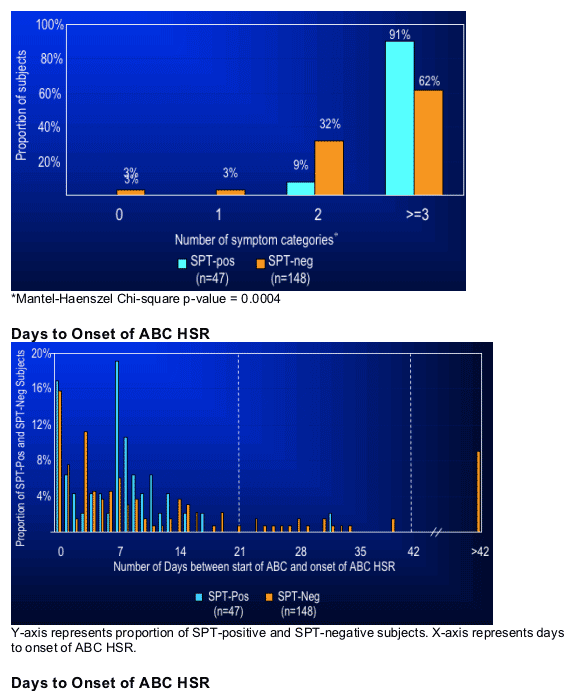
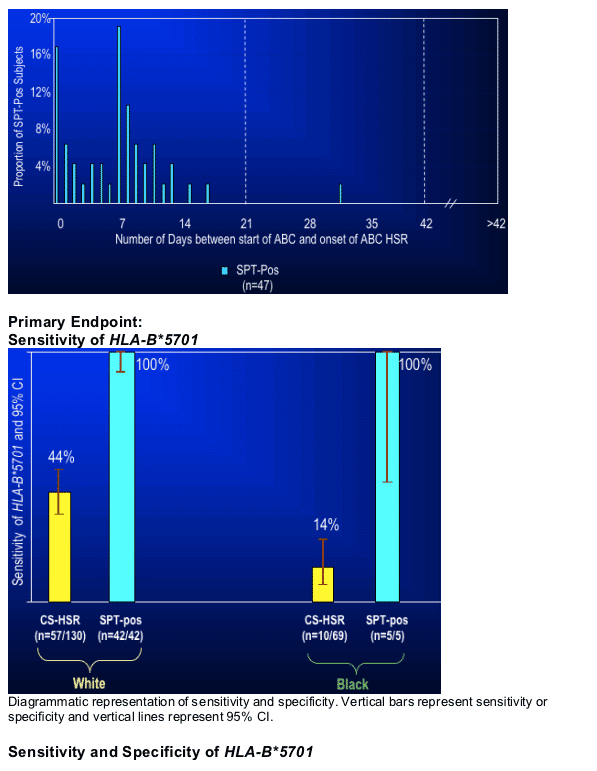
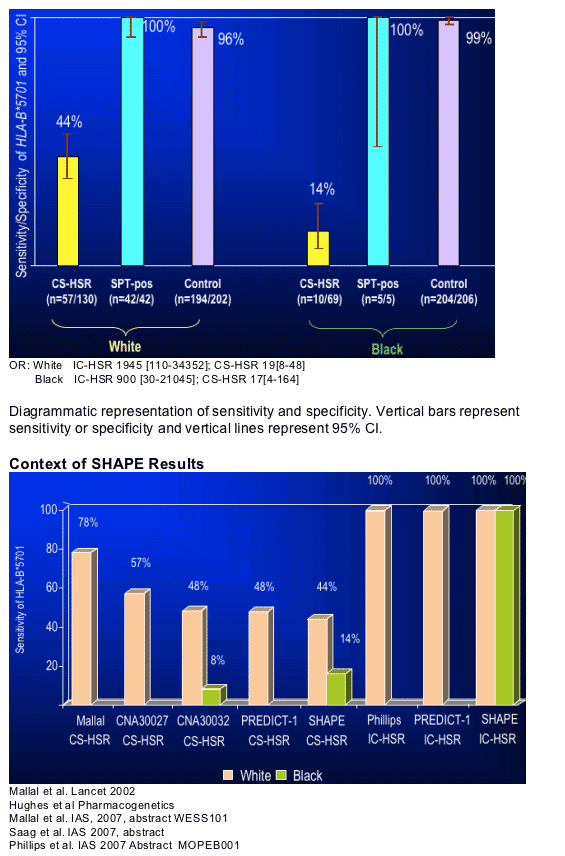
When clinical definition alone was used to define ABC HSR, the results from this study were not unlike those of other studies that used clinically-defined ABC HSR. When abacavir skin patch testing was used to refine the definition, the sensitivity of HLA-B*5701 was 100% not just in Whites, but in Blacks as well.
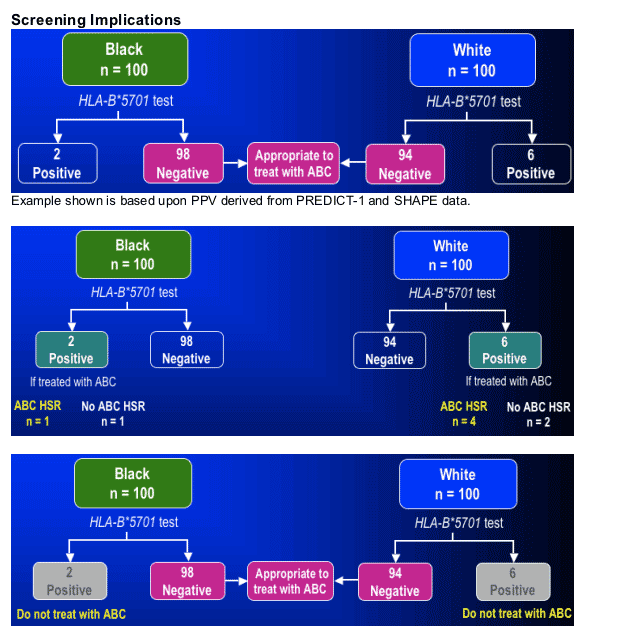
Test 100 Black patients:
--Treat 98 patients at low risk for ABC HSR
--Prevent 1 ABC HSR event
--Exclude ABC in 1 patient
Test 100 White patients:
--Treat 94 patients at low risk for ABC HSR
--Prevent 4 ABC HSR events
--Exclude ABC in 2 patients
|
| |
|
 |
 |
|
|