 |
 |
 |
| |
A randomized study of tenofovir containing HAART compared to lamivudine containing HAART in antiretroviral naive HIV/HBV coinfected patients in Thailand: 48 week findings
|
| |
| |
Reported by Jules Levin
4th IAS Conference, 22-25 July, Sydney, Australia
Matthews GV, Avihingsanon A, Lewin SR, Amin J, Rerknitmitr R, Petcharat P, Marks P, Sasadeusz J, Cooper DA, Bowden S, Locarnini S, Ruxrungtham K, Dore GJ
The prevalence of chronic HBV in HIV-infected individuals is estimated at 5-10% and maybe higher in Southeast Asia where endemic HBV rates are significantly higher and there are high rates of progression to significant liver disease. Lamivudine while active against HBV and HIV can result in high rates of drug resistance to HBV when used for extended periods. This is a randomised clinical trial to compare the safety and efficacy in ART-naive individuals of 3TC, tenofovir, and 3TC-tenofovir combination HAART in HBV/HIV coinfected patients. 36 patients are randomised to 1 of 3 arms and followed for 48 weeks. The first arm contained a backbone of AZT/3TC, the second arm contained AZT/TDF, and the third arm contained combination of TDF+3TC, with efavrienz as the third component in the regimen. 21 patients participated in vk 12-week substudy and these results were previously reported.
AUTHOR CONCLUSIONS
Combination TDF/LAM did not demonstrate superiority to monotherapy in this study, but the proportion of patients with HBV DNA > 3 log (1000) c/ml at week 48 was significantly greater in LAM monotherapy arm, suggesting concerns about drug resistance
There were 2 cases of LAM resistance both in GP 1
Good HIV control was achieved and maintained in the majority
Both seroconversion and hepatic flare common after HBV-active HAART in this population, so its important to monitor patients closely.
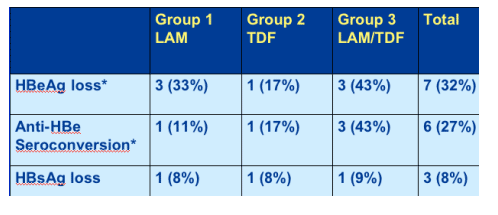
Overall the serological response was good with 32% HBeAg loss rate, 27% anti-HBe seroconversion, and 8% HBeAg loss.
STUDY ENDPOINTS
Primary endpoint
HBV DNA time-weighted change over 48 weeks (DAVG)
Secondary endpoints
HBV DNA suppression
HBeAg/HBsAg seroconversion
HBV resistance
Hepatic flare events
ALT normalisation
Change in HIV-RNA and CD4+ /CD8+ counts
Histological improvement/reduction in ccc-HBV-DNA (for patients who underwent a liver biopsy).
STUDY SET-UP
Australia-Thai collaboration
--Subject recruitment through HIV-NAT, Bangkok, Thailand
--Study management NCHECR, Australia
--HBV virology performed at VIDRL, Australia
HBV virology
--Bayer Versant bDNA assay (2,000 - 108 copies/ml)
--Roche Taqman assay (LLD 200 c/ml)
STUDY ELIGIBILITY
Inclusion criteria
HIV positive and ARV naive
HBsAg positive for > 6 months
HBV DNA > 105 copies/ml
No prior exposure to anti-HBV agents (excl IFN)
Exclusion criteria
HCV-RNA positive or Anti-HAV IgM positive
Acute hepatitis (serum ALT > 1000 U/L)
Active opportunistic infection
Pregnancy or lactation
Concurrent malignancy
Decompensated or Child's C cirrhosis
AFP > 3X ULN
Baseline characteristics
Mean age, 36; one-third female; BMI relatively low at 20-21. Overall 19% had prior AIDS, median CD4 count at baseline 36, HIV RNA 4.7 logs. 78% heterosexual risk factor for HIV.
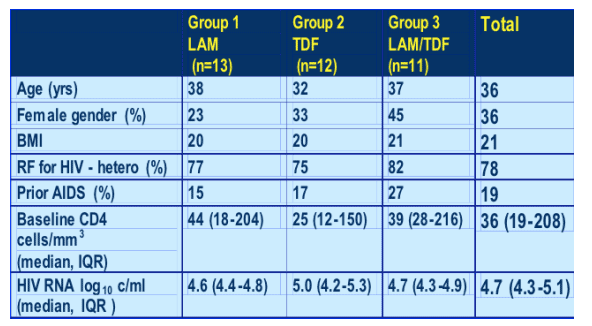
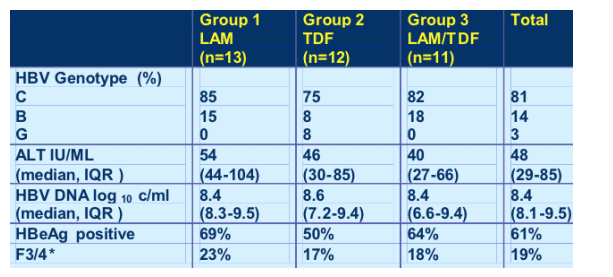
Baseline characteristics : HBV
ALT was normal; HBV DNA was high however, 8.4 logs; 61% were HBeAg+; 19% F/3/4 cirrhosis.
*Clinical or histological diagnosis
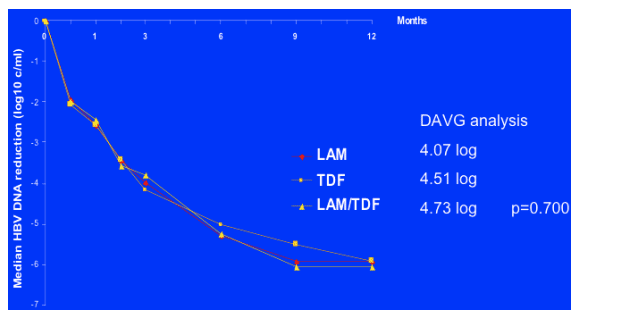
Median Reduction in HBV DNA Week 48
This graph demonstrates the median HBV reduction over the 48 weeks in the 3 arms and visually you can see there is little difference between the groups. This is confirmed by the DAVG analysis which wwasth primary endpoint, demonstarting no significant difference in the values between groups although it was highest in the combination group. Median reduction by the DAVG Primary Study Endpoint analysis over 48 weeks was “greatest in the combination arm”: 4.73 log for LAM/TDF, 4.51 log for TDF, 4.07 for LAM.
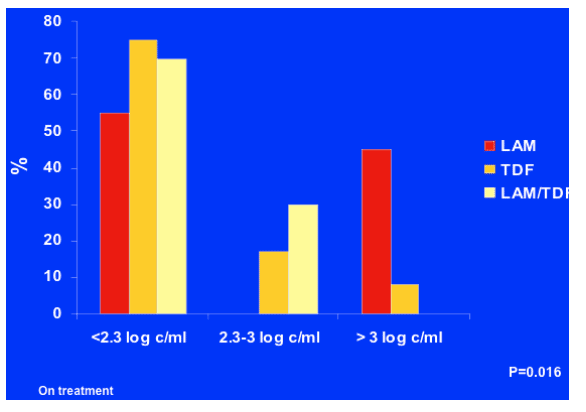
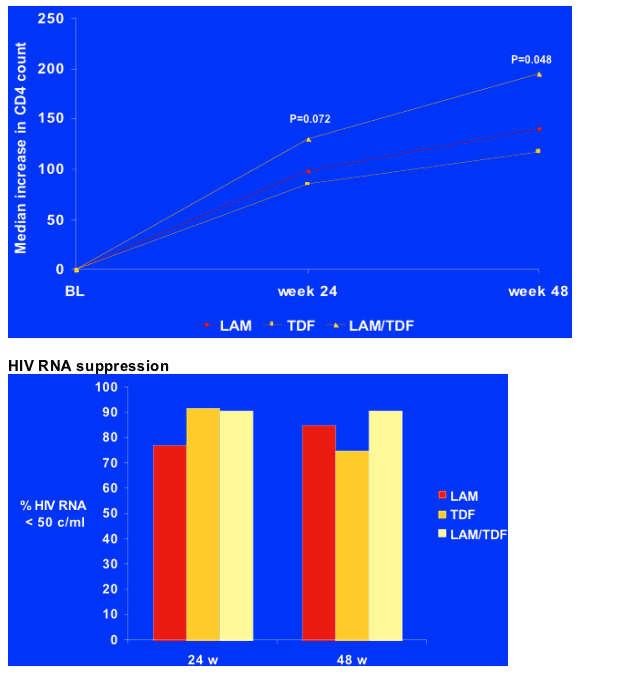
HBV DNA suppression at 48 w: combination is superior
When looking at actual viral suppression, LAM reduced HBV DNA to below 200 copies (<2.3 c/ml) in about 55%, while TDF and LAM/TDF did so in about 70%. Reduction to 200-1000 c/ml was about 20% for TDF and 30% for TDF/LAM. Patients with >1000 c/ml (>3 logs) was 50% for LAM and 10% for TDF. Of note, no patients in the combination TDF/LAM arm failed to reduce viral load to <1000 c/ml and 1 patient in the TDF arm failed to reduce viral load to <1000 c/ml, and 5 patients or 45% in the LAM failed to reduce viral load to <1000 c/ml.
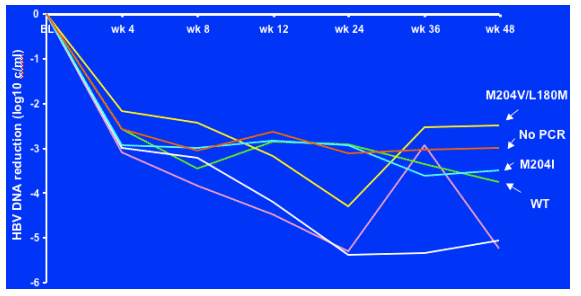
HBV resistance
This is a graph of the 6 patients who failed to reach <1000 c/ml by week 48 and you can see the HBV drug resistance developed. 2/5 patients displayed resistance (M204V/L,180M, M204I—LAM resistance), wild-type in 1 patient, for 1 patient no pcr was available. It appears that the TDF patient did not display resistance.
CD4
Median CD4 increase was highest in the combination TDF/3TC arm although it was good in all arms.
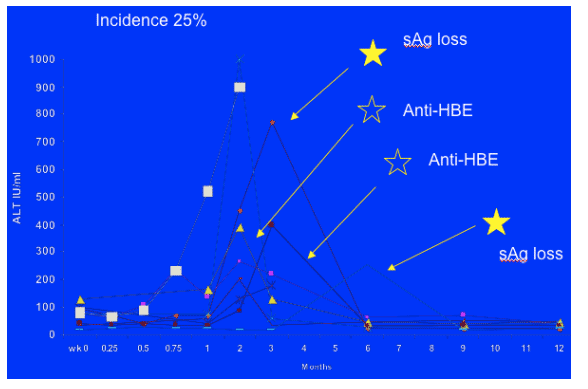
Hepatic flare
(defined as ALT 5x ULN or rise of 200 units rise from baseline if baseline was abnormal)
There were 9/36 patients (25%) with hepatic flare, and 8/9 it occurred within the first 12 weeks. One patient had baseline median CD4 count of 70 and had cirrhosis at baseline experienced rapid decompensation and died. Other patients LFTs returned to normal by the end of the study. Reasons for hepatic flare in patients with HIV HBV who initiate HAART may be multifactoiral, and include drug toxicity, flare in HBV disease and immune reconstitution disease. In our patients hepatic flare was followed by seroconversion to anti-HBE in 2 (I think she said 4 seroconverted and S ag loss in 2 patients) and to anti-HBe followed by anti-HBs in two.
Serological response
*Of 22 HBeAg positive at BL
|
| |
|
 |
 |
|
|