 |
 |
 |
| |
LPV/r-based 2-drug HAART vs LPV/r-based 3-drug HAART: Comparable virological efficacy and tolerability in HIV-1-infected naive subjects (Kalead1 study) – 48-Week results
|
| |
| |
Reported by Jules Levin
4th IAS Conference on HIV Pathogenesis, Treatment and Prevention, Sydney,
Australia, 22-25 July 2007
Pinola M.1, Carosi G.2, Di Perri G.3, Lazzarin A.4, Moroni M.5, Vullo V.6, Leoncini F.7, Mazzotta F.8, Pastore G.9, Norton M.10, Di Luzio Paparatti U.1, for the Kalead1 Study Group
1Abbott S.p.A., Campoverde (LT), Italy - 2University of Brescia, Brescia, Italy - 3University of Torino, Turin, Italy - 4San Raffaele Hospital, Milan, Italy - 5University of Milan, Milan, Italy - 6University of Rome 'La Sapienza', Rome, Italy-7Careggi Hospital, Florence, Italy - 8S. Maria Annunziata Hospital,
Firenze, Italy - 9Policlinico Consorziale Hospital, Bari, Italy - 10Abbott Laboratories, North Chicago (IL), United States
Author Conclusions
48-week results demonstrated comparable virologic efficacy and tolerability when a dual regimen of LPV/r + TDF was compared to LPV/r + 2 NRTIs in ARV-naive patients. CD4 increases were significantly greater in the dual therapy arm at Week 48. Finally, while not reaching statistical significance, there was a trend for lower incidence of lipid elevations in the dual therapy arm.
Author Discussion
Kalead1 is the first study to compare dual ART therapy with standard-of-care triple therapy in HIV-infected adults naive to ARV therapy. The results of the Kalead1 study suggest that a dual therapy with LPV/r + TDF may be equally effective and tolerable, when compared to threedrug HAART.
Monotherapy strategy with LPV/r has been compared to triple therapy with LPV/r + 2 NRTIs employing various approaches (ARV naive, induction- maintenance, simplification to LPV/r monotherapy among virologically suppressed). LPV/r monotherapy while effective, does carry an increased risk of selecting protease resistance mutations, a greater incidence of viral blips and viral failure when compared to triple therapy with LPV/r as the anchor. In our Kalead1 study, through 48 weeks, there was no difference between the dual and the triple arm with regard to the incidence of viral blips, emergence of PI resistance, nor virologic failure.
The limitations of Kalead1 study include the choice to use Investigator-selected NRTIs in the triple arm instead of a standard comparative arm. Also, the dosing of LPV/r was not QD in neither of the treatment arms, due to the lack of QD labelling in Italy. Obviously the attractiveness of dual therapy would be enhanced if the LPV/r was dosed QD, thereby making the entire regimen QD. Furthermore, the new tablet formulation of LPV/r was only used in the Kalead1 study by a few subjects near the end of the trial (none prior to week 48).
Further study of dual therapy HAART with LPV/r employing QD dosing, with the tablet formulation, and with other NRTI partners (3TC, FTC, ABC) is warranted.
BACKGROUND
Current guidelines for the management of HIV-1 infected subjects recommend the use of 3-drug combination therapy. The use of 3-drug HAART may not always be feasible. Patients can have specific contraindications that make the incorporation of 3 drugs difficult.
They may have drug resistance that can reduce the antiviral activity of a drug in the 3 drug regimen. Therefore a rationale exists to consider drug regimens using less than 3 drugs.
A virologically suppressive 2-drug regimen, which does not harbour an increased risk of drug resistance, which reduces the economic cost of requiring a third drug for HIV treatment, and eliminates any toxicity associated with a third drug, would be attractive for the treatment of HIV-1 infection. Kalead1 is the first study of the combination of Lopinavir/ritonavir soft gel capsules (LPV/r SGC) and Tenofovir DF (TDF) as first-line HAART.
Objectives
Kalead1 was designed to compare the antiviral activity and safety of the combination of LPV/r SGC and TDF as first-line 2-drug regimen, versus a standard-of-care 3-drug regimen of LPV/r SGC in combination with 2 nucleoside analogues.
METHODS
Study Design
Kalead1 is a 72-week, phase III, open-label, randomized, comparative, multi-center trial comparing the antiviral efficacy of two-drug therapy with LPV/r in combination with TDF versus the SOC three-drug therapy (LPV/r in combination with 2 NRTI’s) in antiretroviral naive, HIV-1 infected adults.
The study consists of two phases. The first phase of 24 - 26 weeks was built into the study design to gauge initial virologic safety of a 2-drug regimen. If virologic suppression was successful during Phase 1 (HIV-RNA <50c/mL), then the subject continued in the trial for further 48 weeks of Phase 2. (Fig. 1)
Antiretroviral naive male and female subjects, >18 years of age, with plasma HIV-1 RNA > 400 copies/mL and any CD4 count were eligible for study entry.
The subjects were randomized to receive LPV/r SGC 400/100 mg BID + TDF 300 mg QD or LPV/r SGC 400/100 mg BID + 2 NRTIs chosen by the Investigator. The subjects were monitored for plasma HIV-1 RNA, CD4 cell count, adverse events and routine clinical laboratory studies.
All randomized subjects who received at least one dose of study drug are included in this interim 48-weeks efficacy analysis for noninferiority.
Baseline data
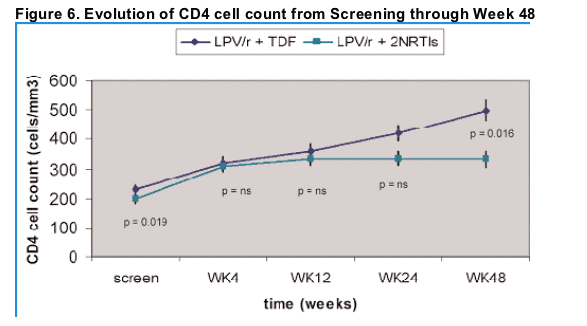
The two treatment arms were comparable at baseline for all criteria except CD4 cell count. CD4 count was significantly lower (p=0.019) in the Triple Arm (LPV/r + 2NRTIs). The difference between the two treatment arms was no longer evident when the subjects were stratified for CD4 cell counts < 200 cells/mm3 vs >200 cells/mm3 (p = ns).
Table 1. Baseline data
Only CD4 cell count difference was significant.
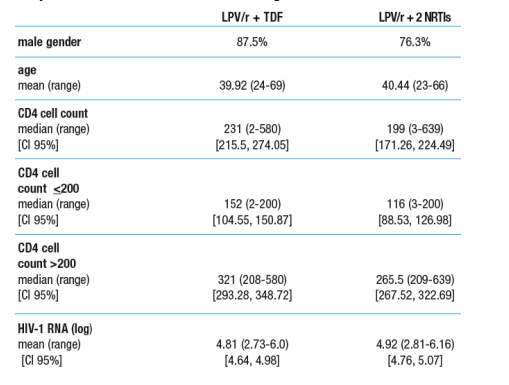
72 subjects were randomized to LPV/r+TDF (Dual Arm) and 80 to LPV/r+2NRTIs (Triple Arm). 62/72 (86.1%) and 63/80 (78.8%) of the subjects in Dual Arm and Triple Arm, respectively, reached the end of phase 1.
51 (82.3%) and 56 (88.9%) of the subjects who reached the end of Phase 1 did so with two consecutive HIV RNA values below 50 copies and thus qualified to enter Phase 2 of the study.
Of the 51 subjects in the dual and 56 subjects in the triple arm, further 7 (13.7%) and 6 (10.7%) subjects discontinued before Week 48
Figure 2. Kalead1 Subject Summary
There were 10 discontinuations out of 72 started on LPV/r+TDF, and 17 discontinuations from triple regimen. Of the 62 subjects in the double arm at week 24/26, 11 were not virologically suppressed. And there were 44 subjects in the 2-drug arm at week 48, of which 38 had <50 c/ml. Of the 63 on triple therapy at week 24/26, 7 were not virologically suppressed. Of the 50 subjects at week 48, 42 were <50 c/ml. The difference in % <50 c/ml at week 48 was not significant.
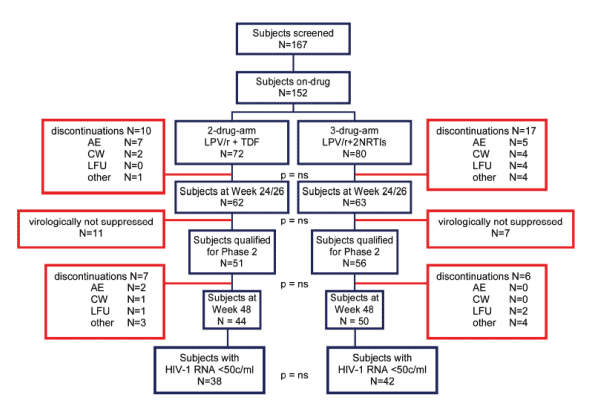
The distribution of the Investigator-prescribed NRTIs in the Triple Arm (LPV/r + 2NRTIs) is presented in Figure 3.
Figure 3. Investigator-prescribed NRTI combinations in the 3-drug-arm
64% AZT/3TC, 26% 3TC/ABC, 4% ABC/FTC, 3% 3TC/ddI, 3% 3TC/d4T.
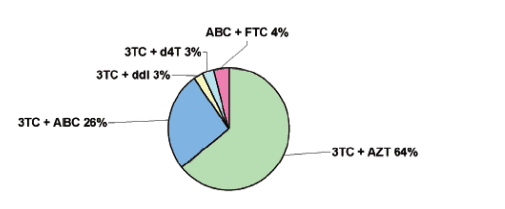
Efficacy
The reduction in HIV-1 RNA from baseline to Week 48 was comparable between the two treatment arms. (Fig. 4).
The proportion of subjects with viral load <400 copies/mL and <50 copies/mL from Screening through Week 48 in Intention-To-Treat (ITT) and On-Treatment (OT) populations is presented in Figure 5, Panel 1 & 2, respectively:
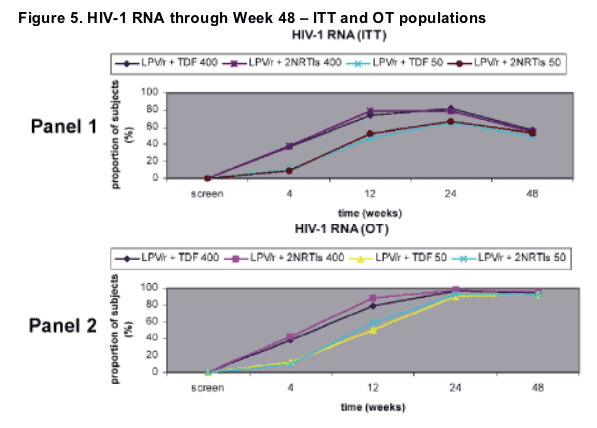
5 subjects (6.94%) in the dual arm had a viral blip at Week 48, the values ranging from 51 to 91 copies/mL, whereas in the triple arm the viral blips were 3 (3.75%; p=ns), ranging from 72 to 1000 copies. No resistance testing was performed.
The change in CD4 counts through Week 48 is presented in Figure 6.
Safety
Discontinuations
The number of discontinuations due to various reasons was comparable in the two treatment arms through Week 48 (see Fig. 2 for details).
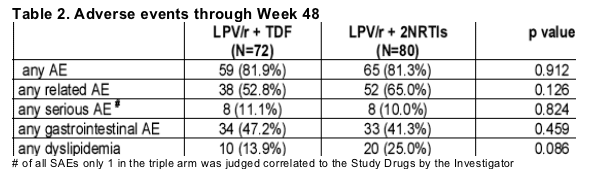
Adverse Events
The incidence of adverse events through Week 48 was comparable in the two groups, except Investigator-reported lipid elevations (dyslipidemia, hyperlipidemia, hypercholesterolemia, hypertriglyceridemia) which were less frequent in the dual arm (p=0.086).
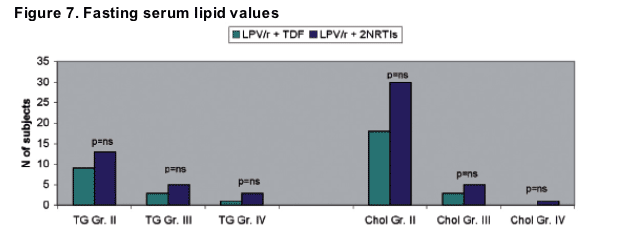
When laboratory values of fasting serum lipids were compared, the differences between Grade II-III-IV elevations of triglycerides and total cholesterol were not significant between the two treatment arms (Fig. 7).
References
1. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. DHHS, October 10, 2006.
2. JF Delfraissy et al. MONARK Trial (MONotherapyAntiRetroviral Kaletra): 48-Week Analysis of Lopinavir/Ritonavir (LPV/r) Monotherapy compared
to LPV/r + Zidovudine/Lamivudine (AZT/3TC) in Antiretroviral-Naive Patients. AIDS 2006 – XVI International AIDS Conference: Abstract n. THLB0202
3. W Cameron et al. A two-year randomized controlled clinical trial in antiretroviral-naive subjects usine lopinavir/ritonavir (LPV/r) monotherapy
after initial induction treatment compared to an efavirenz (EFV) 3-drug regimen (Study M03-613). AIDS 2006 – XVI International AIDS Conference: Abstract n. THLB0201
4. J Arribas et al. Lopinavir/ritonavir as single-drug maintenance therapy in patients with HIV-1 viral suppression: Forty-eight week results of a
randomized, controlled, open label, clinical trial (OK 04 Study). AIDS 2006 – XVI International AIDS Conference: Abstract n. THLB0203
5. C Delaugerre et al. Protease Gene Mutations in a Trial Comparing First Line LPV/r Monotherapy to LPV/r + AZT/3TC (MONARK Trial). XVI International HIV Drug Resistance Workshop: Poster n. 75
|
| |
|
 |
 |
|
|