 |
 |
 |
| |
Efficacy and Safety of Darunavir/ritonavir versus Lopinavir/ritonavir in ARV Treatment-Naive HIV-1-Infected Patients at Week 48:
ARTEMIS (TMC114-C211)
|
| |
| |
DeJesus E, Ortiz R, Khanlou H, Voronin E,
Van Lunzen J, Andrade-Villanueva J, Fourie J,
De Meyer S, Haley M, Lefebvre E,
Vanden Abeele C, Spinosa-Guzman S
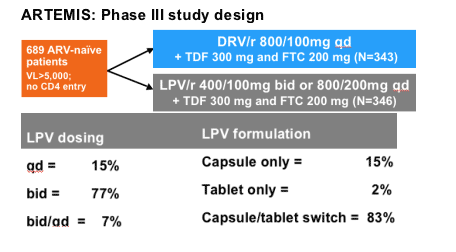
Dosing was based on regulatory approval; switch was made
according to local regulatory approval and drug availability
AUTHOR CONCLUSIONS
The use of once-daily DRV/r 800/100mg + TDF/FTC in
treatment-naive patients:
--resulted in excellent virologic and immunologic responses
--provided suitable exposure in all patients
--was well tolerated, with a favorable safety profile
In comparison to the LPV/r arm* in treatment-naive patients:
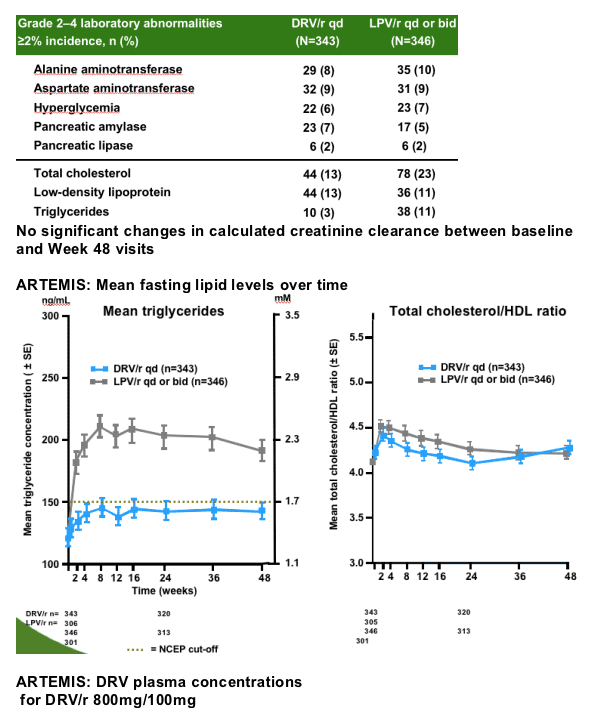
--For efficacy, DRV/r 800/100mg qd was non-inferior in the overall population, and superior in patients with high VL
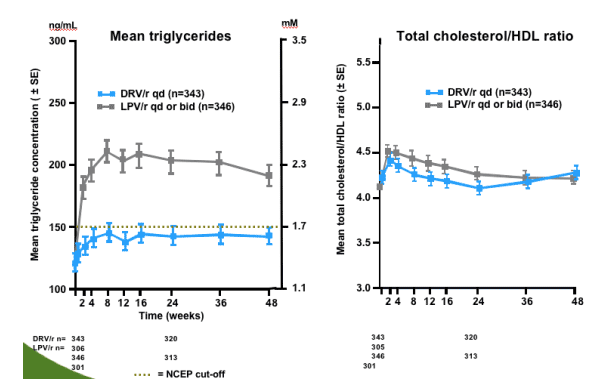
DRV/r had lower incidence of common GI toxicities and triglyceride elevations
*LPV/r arm included: LPV/r 400/100mg bid or 800/200mg qd, capsule and tablet formulations
ARTEMIS: Study objectives
Primary end point
--Proportion of patients with an HIV RNA <50 copies/ml at Week 48
Primary objective
--demonstrate non-inferiority of DRV/r qd vs LPV/r based on that primary end point
Secondary objectives
--evaluate long-term safety, tolerability and durability of virologic responses
--compare immunologic responses conduct pharmacokinetic evaluations
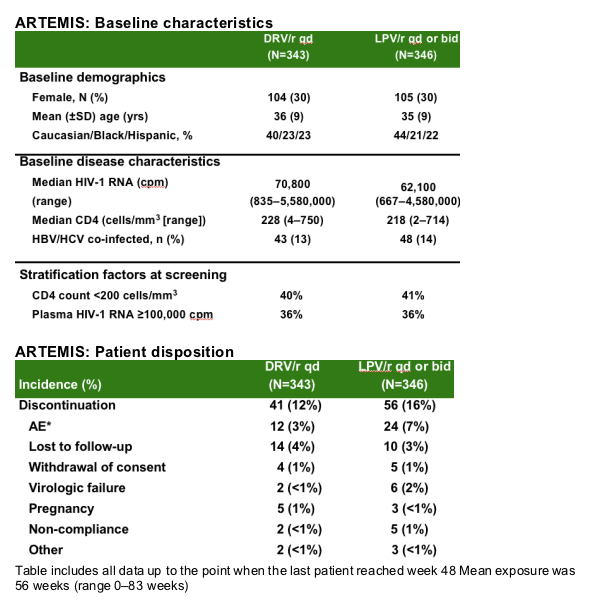
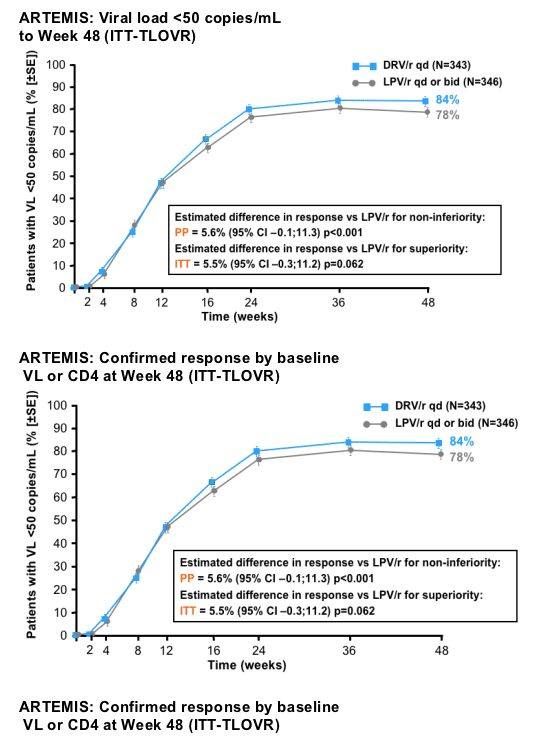
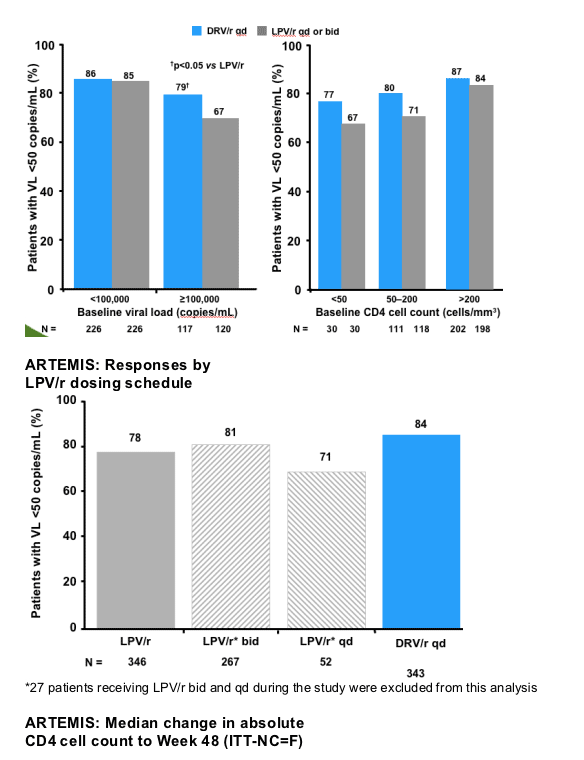
|
| |
|
 |
 |
|
|