 |
 |
 |
| |
Pluses and Minuses of First-Line Lopinavir Versus Efavirenz: drug resistance in ACTG 5142
|
| |
| |
XVI International HIV Drug Resistance Workshop
June 12-16, 2007
Barbados
Mark Mascolini
A first-line efavirenz regimen may have a virologic advantage over up-front lopinavir/ritonavir, according to previously reported results of AIDS Clinical Trials Group (ACTG) study 5142 [1]. But that plus must be weighed against a resistance minus with efavirenz, a Resistance Workshop study found [2]. Further analysis of ACTG 5142 also provided another reason to be wary of first-line efavirenz plus lopinavir/ritonavir and no nucleosides: This combination poses an even greater resistance threat than the standard three-drug combos.
At the 2006 International AIDS Conference, ACTG 5142 investigators reported a significantly swifter time to virologic failure in 96 weeks with lopinavir/ritonavir plus two nucleosides than with efavirenz and two nucleosides [1]. That report brushed out some of the broad strokes of the resistance disadvantages with efavirenz plus either nucleosides or lopinavir/ritonavir. At the Resistance Workshop, Richard Haubrich (University of California, San Diego) dabbed in the fine details after a median 112 weeks of follow-up.
The ACTG team enrolled 753 antiretroviral-naive people with a median CD4 count of 191 and a median viral load of 4.8 log and assigned them to one of three regimens: (1) efavirenz (600 mg daily) plus lopinavir/ritonavir (533/133 mg twice daily), (2) efavirenz (600 mg daily) plus two nucleosides, or (3) lopinavir/ritonavir (400/100 mg twice daily) plus two nucleosides. Nucleoside duos consisted of 3TC plus either AZT, d4T, or tenofovir.
Defining virologic failure as an early viral load rebound or a load above 200 copies after study week 32, Haubrich confirmed the significantly superior results with efavirenz plus nucleosides than with the protease inhibitors (PIs) plus nucleosides (Table).
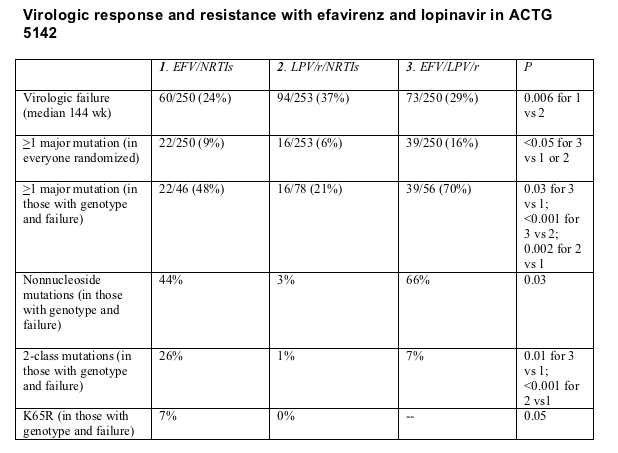
Among everyone randomized in the trial, at least one major mutation arose significantly more often in people taking the nucleoside-sparing regimen of efavirenz plus the PIs (16%) than in those taking either efavirenz plus nucleosides (9%) or lopinavir/ritonavir plus nucleosides (6%) (Table). When Haubrich limited the analysis to people who got genotyped for resistance and had virologic failure, the rate of at least one major mutation looked even worse with efavirenz plus the PIs (70%) than with the standard triple regimens including efavirenz (48%) or lopinavir/ritonavir (21%). In this analysis lopinavir/ritonavir plus two nucleosides yielded significantly fewer major mutations than efavirenz plus two nucleosides.
The no-nuke efavirenz/PI combo also looked bad when Haubrich compared the number of nonnucleoside mutations that arose with failure of that regimen (66%) and the number of mutations after failure of the standard efavirenz/nucleoside threesome (44%). But efavirenz plus two nucleosides proved the riskiest regimen when comparing rates of two-class resistance upon failure or rates at which K65R emerged. In this analysis two-class resistance meant at least one mutation to any drug in a class, not resistance to every drug in the class.
Haubrich also saw a trend toward fewer nucleoside mutations of any type with lopinavir/nucleosides (19%) than with efavirenz/nucleosides (30%) (P = 0.19). M184V and thymidine analog mutations arose at similar frequencies with efavirenz/nucleosides and lopinavir/ritonavir/nucleosides, 17% among those with virologic failure and genotype.
Why nonnucleoside mutations cropped up so much more often with failure of efavirenz/lopinavir than with failure of efavirenz plus two nucleosides remains unclear. Haubrich said the ACTG definitively ruled out one possibility--differing adherence between the two groups. He speculated that, despite the PI dose adjustment in the lopinavir/efavirenz arm, efavirenz levels may still have been lower in this group than in the efavirenz/nucleosides group.
References
1. Riddler SA, Haubrich R, DiRienzo G, et al. A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV infection--ACTG 5142. XVI International AIDS Conference. August 13-18, 2006. Toronto. Abstract THLB0204.
2. Haubrich RH , Riddler SA, DiRienzo AG, et al. Drug resistance at virological failure in a randomized, Phase III trial of NRTI-, PI- and NNRTI-sparing regimens for initial treatment of HIV-1 infection (ACTG 5142). Antiviral Therapy 2007;12:S66. Abstract 57.
|
| |
|
 |
 |
|
|