 |
 |
 |
| |
Three Years of Entecavir (ETV) Re-treatment of HBeAg(-) ETV Patients Who Previously Discontinued Treatment: Results from Study ETV-901
|
| |
| |
Reported by Jules Levin
AASLD Nov 4 2008 San Francisco, CA
Daniel Shouval1, Ching-Lung Lai2, Ting-Tsung Chang3, Adrian Gadano4, Shun-Sheng Wu5, Waldemar Halota6, William Sievert7, Naoky Tsai8, Hui Zhang9, Uchenna Iloeje9
1Liver Unit, Hadassah Medical Organization, Jerusalem, Israel; 2Department of Medicine, Queen Mary's Hospital, University of Hong Kong, Hong Kong; 3Department of Internal Medicine, National Cheng Kung University Medical College, Tainan Taiwan; 4Hospital Italiano, Buenos Aires, Argentina; 5Department of Internal Medicine, Changhua Christian Hospital,
Changhua, Taiwan; 6Klinika Chorob Zakaznch AM, Bydgoszcz, Poland; 7Department of Medicine, Monash Medical Centre, Monash University, Melbourne, Australia; 8University of Hawaii, Honolulu, USA; 9Research and Development, Bristol-Myers Squibb Company, Wallingford, USA
AUTHOR SUMMARY
ETV-027 demonstrated that discontinuation of effective antiviral therapy in HBeAg(-) patients after 1 year of treatment results in rebound of viremia and increases in ALT
Three years of ETV re-treatment in this cohort resulted in:
-- 95% achieving HBV DNA <300 copies/mL
-- 86% achieving ALT <1 x ULN
-- Of the patients who discontinued therapy, the majority (24/32; 75%) had HBV DNA <300 copies/mL
The safety profile remains consistent with previous reports
AUTHOR CONCLUSION
ETV re-treatment of HBeAg(-) CHB patients who discontinued antiviral therapy, resulted in HBV DNA suppression and ALT normalization similar to that achieved prior to treatment discontinuation
Observations from this cohort demonstrate that long-term treatment with ETV results in durable suppression of HBV DNA replication
Durable suppression with long-term ETV results in regression of fibrosis/cirrhosis (see Poster 894)
INTRODUCTION
Entecavir (ETV) 0.5 mg daily demonstrated superior virologic, histologic and biochemical activity compared to lamivudine (LVD) 100 mg daily in nucleoside-na´ve HBeAg(-) patients (study ETV-027)1
At Week 48, the majority of patients treated in ETV-027 met the protocol-defined criteria of 'Response' and discontinued therapy after Week 52
During off-treatment follow-up, most of these patients experienced recurrent viremia and increases in alanine aminotransferase (ALT)
Results from ETV-027 demonstrated that 1 year of treatment with a potent nucleoside analogue is insufficient to achieve sustained suppression of HBV DNA replication
One-hundred and eleven ETV-treated patients from ETV-027 enrolled in rollover study ETV-901 (1.0 mg ETV daily)
ETV-901 provides the opportunity to evaluate the effect of long-term ETV re-treatment in patients who previously discontinued therapy
Methods
Study population
The HBeAg(-) ETV Re-treatment Cohort consists of patients who:
- were initially treated with ETV in ETV-027
- subsequently enrolled in ETV-901 with a >60 day treatment gap between ETV-027 and ETV-901
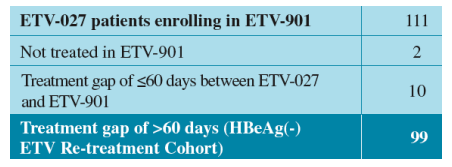
The HBeAg(-) ETV Re-treatment Cohort is an observational cohort that was defined without regard to:
- treatment response at end of dosing in ETV-027
- HBV DNA or ALT measurements at the start of dosing in ETV-901
Initially, due to ongoing blinding of Phase 2-3 studies, patients enrolling into study ETV-901 received a combination of ETV 1.0 mg and LVD 100 mg daily (the
protocol was amended for patients to receive monotherapy with ETV 1.0 mg daily)
Evaluations
Patients in the HBeAg(-) ETV Re-treatment Cohort were assessed at Weeks 12, 24, 36, 48, 72, 96 and 144 after re-initiation of treatment in ETV-901 (Non-completer = Missing)
Efficacy assessments evaluated the proportion of patients with available samples for the following parameters:
- HBV DNA <300 copies/mL by PCR
- ALT ≦1 x ULN
- HBsAg loss
HBV DNA measurements were performed at a central laboratory; ALT measurements were performed at local laboratories
Direct nucleotide sequencing for resistance testing was conducted on all patients with HBV DNA ≥300 copies/mL at Year 2 or at last observation for patients who discontinued prior to the Year 2 visit
Resistance testing of samples for the Year 3 analyses is pending
Safety was assessed by the incidence of clinical adverse events and laboratory abnormalities
RESULTS
Study population
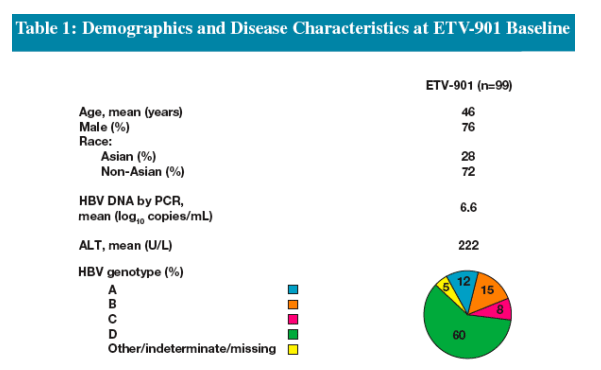
Mean baseline HBV DNA in ETV-901 was 6.64 log10 copies/mL vs. 7.6 log10 copies/mL at baseline in ETV-027 (all treated patients)
Mean baseline ALT was 222 U/L in ETV-901 vs. 141 U/L at baseline in ETV-027 (all treated patients)
Exposure
Seventeen patients received ETV monotherapy only for a mean of 154 weeks (median 157 weeks); 11 patients received ETV and LVD combination therapy only for a mean of 36 weeks (median 48 weeks); 71 patients received ETV and LVD combination therapy for a mean of 32 weeks (median 32 weeks) followed by ETV monotherapy for a mean of 134 weeks
A total of 32 patients discontinued treatment before the Year 3 visit
The reasons for patient discontinuation included:
- completed treatment = 19 (59%)
- patient withdrew = 5 (16%)
- non-compliance = 2 (6%)
- other = 6 (19%)
Among patients who discontinued treatment prior to the Year 3 visit, 24 (75%) had HBV DNA <300 copies/mL on their last PCR measurement
HBV DNA suppression
Ninety-four percent of patients in the HBeAg(-) ETV Re-treatment Cohort had achieved HBV DNA <300 copies/mL by end of dosing in ETV-027
The majority of patients experienced recurrent viremia during the off-treatment follow-up period
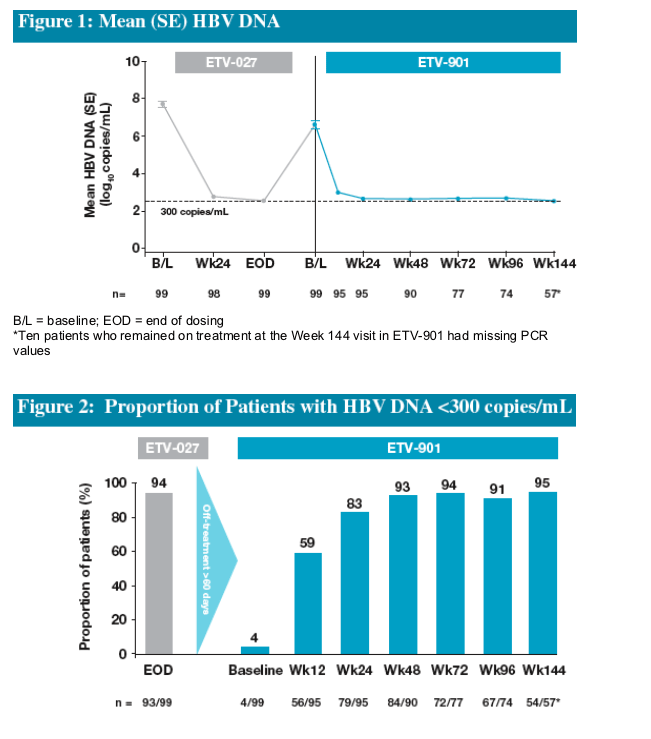
Re-treatment of patients in this cohort resulted in 83% of patients achieving HBV DNA <300 copies/mL by Week 24
Proportions of patients achieving HBV DNA <300 copies/mL increased to 93% by Week 48 and this was maintained on continued treatment through Year 3
ALT normalization
Seventy-eight percent of patients in the HBeAg(-) ETV Re-treatment Cohort achieved ALT <1 x ULN by end of dosing in ETV-027
The majority of patients experienced an increase in ALT towards baseline levels (or higher) during the off-treatment follow-up period
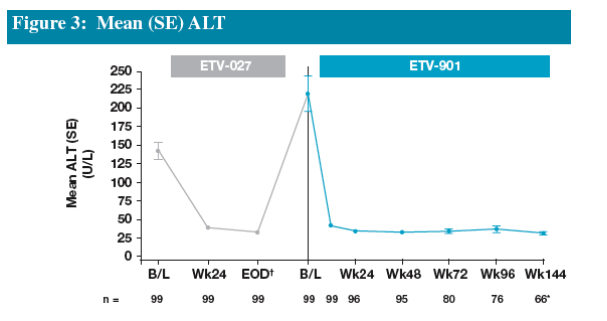
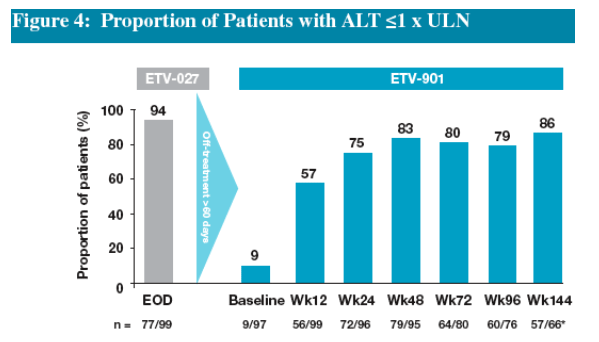
Re-treatment of patients in this cohort resulted in 75% of patients achieving ALT <1 x ULN by Week 24
Proportions of patients achieving ALT <1 x ULN increased to 83% by Week 48 and this was maintained on continued treatment through Year 3
Resistance Analyses
7/74 patients analyzed at Year 2 had HBV DNA ≥300 copies/mL
- Two patients (both with detectable LVD resistance at baseline by nucleotide sequencing) had ETV resistance
· One of these patients was previously reported2
· The second patient was not detected until Year 2 of ETV-901 and had HBV DNA <300 copies/mL at end of dosing in Phase 3 (Note: This patient did not
receive continuous ETV therapy and is not part of the ETV Resistance Cohort)
4/23 patients who discontinued from study prior to the Year 2 visit had HBV DNA ≥300 copies/mL
- All were tested and none had ETVr
3/57 patients analyzed at Year 3 and 4/9 patients who discontinued between Years 2 and 3 had HBV DNA ≥300 copies/mL
- Resistance testing of samples from these seven patients is pending
References
1. Lai CL, Shouval D, Lok AS, et al. N Engl J Med 2006;354:1011-20.
2. Colonno R, Rose R, Baldick CJ, et al. Hepatology 2006;44(6):1656-65.
|
| |
|
 |
 |
|
|