 |
 |
 |
| |
Week 96 Resistance Surveillance for HBeAg Positive and Negative Subjects with Chronic HBV Infection Randomized to Receive Tenofovir DF 300 mg QD
|
| |
| |
Reported by Jules Levin
AASLD Nov 4 2008, San Francisco, CA
A Snow-Lampart1, B Chappell1, M Curtis1, Y Zhu1, J Heathcote2, P Marcellin3, and K Borroto-Esoda1
1Gilead Sciences Inc., Durham, NC, USA; 2University of Toronto, Toronto, ON, Canada; 3University of Paris, Hospital Beaujon, Clichy, France
AUTHOR CONCLUSIONS
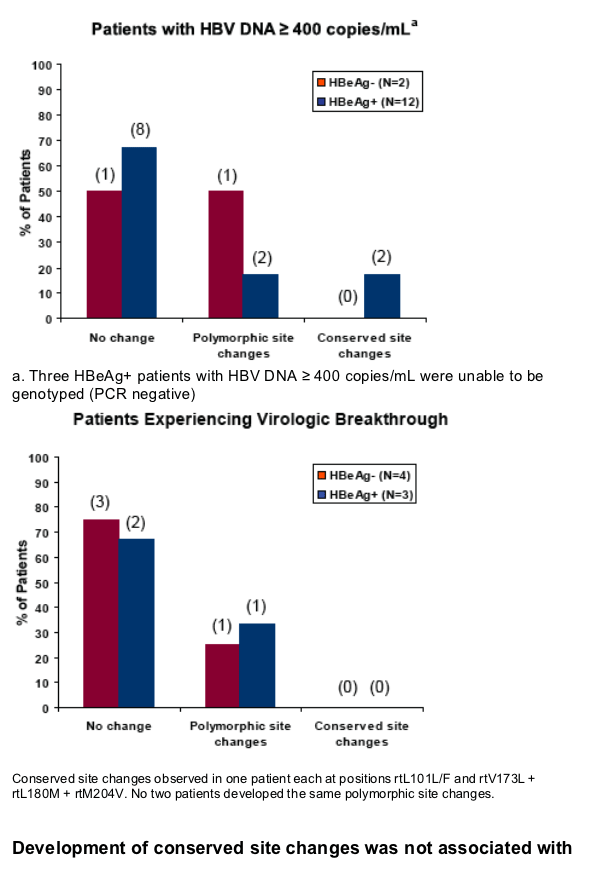
No HBV pol/RT amino acid substitutions associated with resistance to tenofovir were detected through 96 weeks of tenofovir DF mono-therapy
-- Annual resistance surveillance on-going through year 8 (week 384)
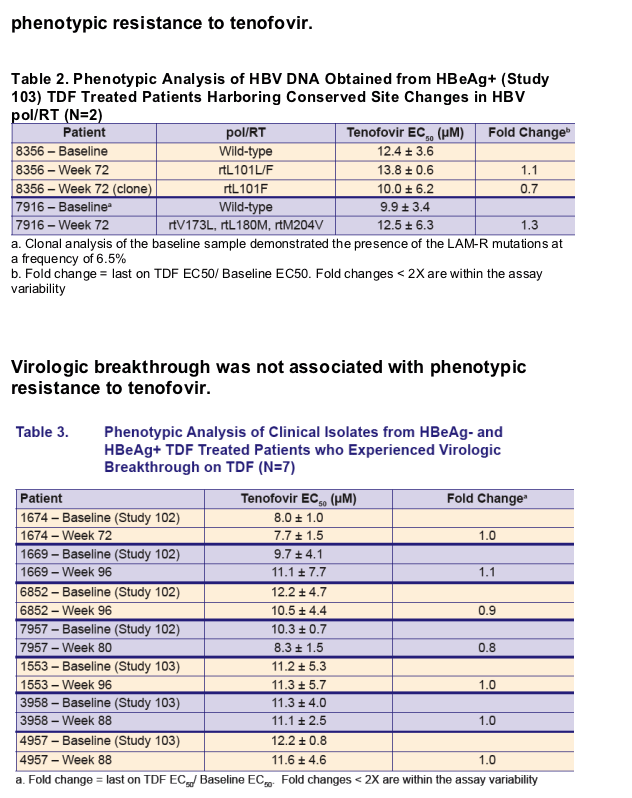
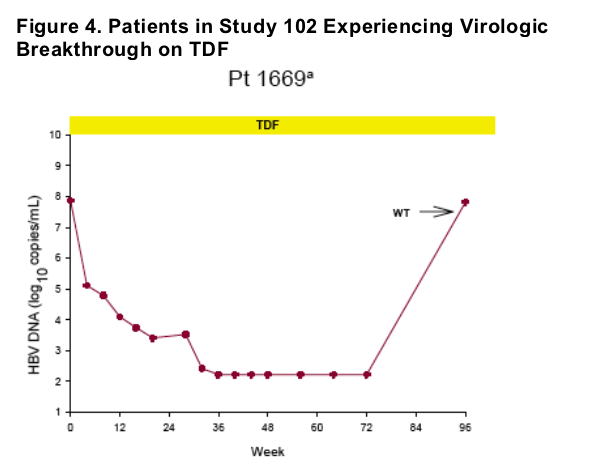
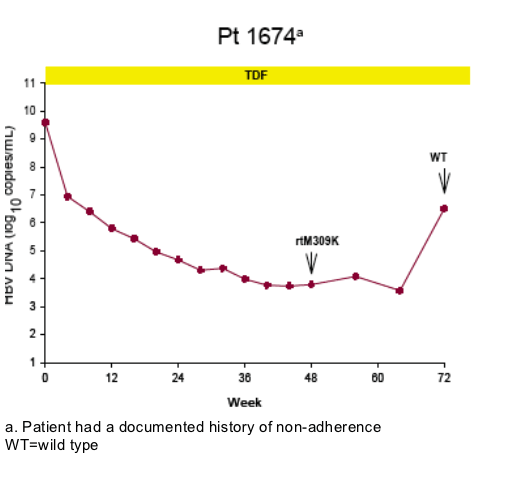
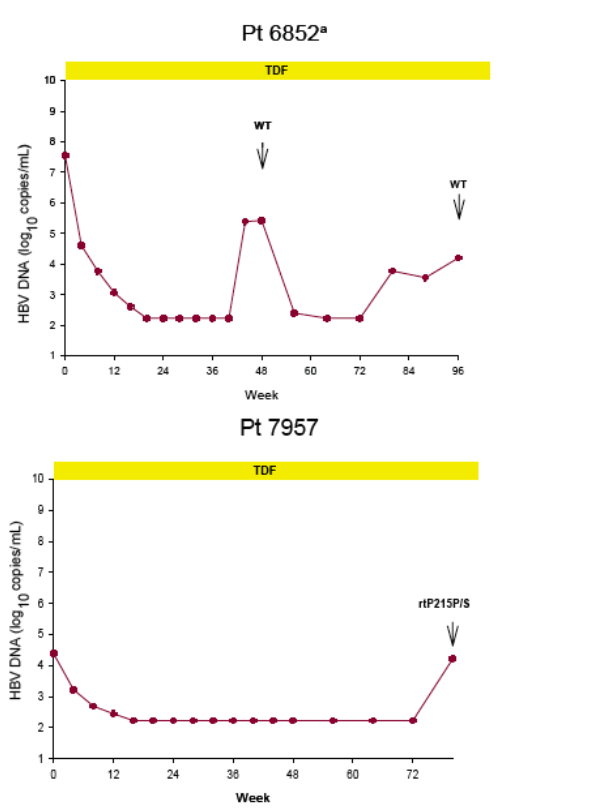
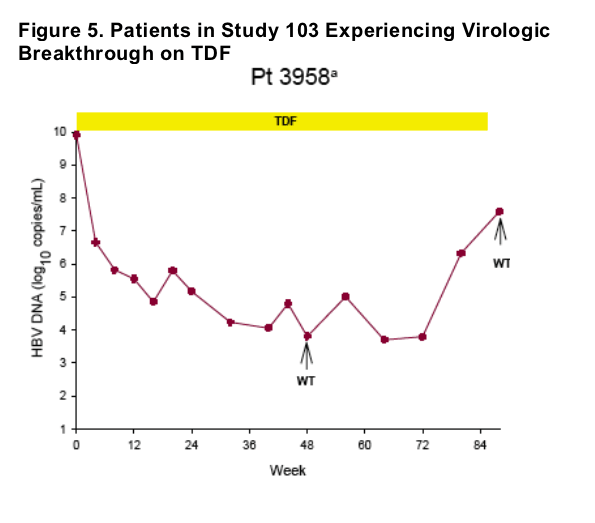
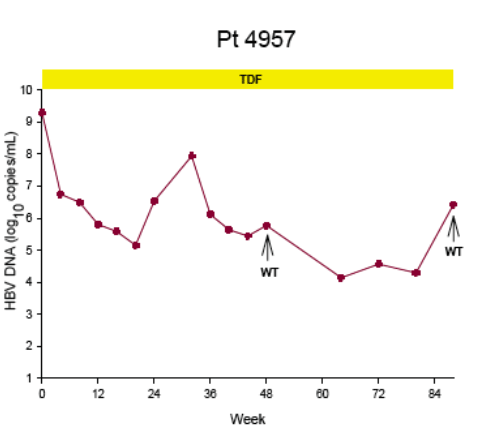
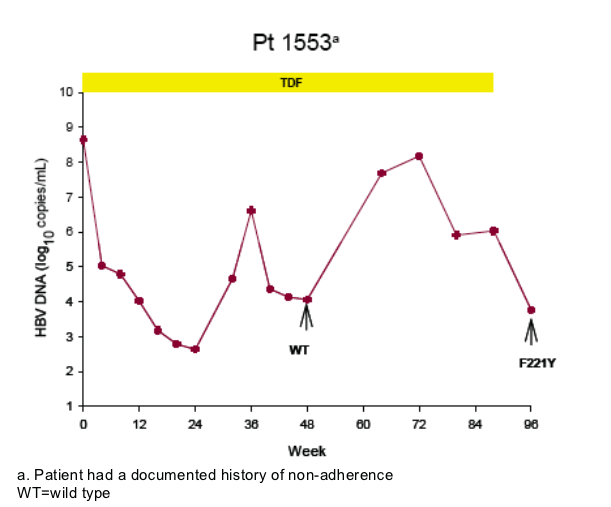
Virologic breakthrough was infrequent and not associated with phenotypic resistance to tenofovir
-- The majority of patients experiencing virologic breakthrough had evidence of
non-adherence
Development of conserved site changes was rare and not associated with phenotypic resistance to tenofovir
INTRODUCTION
Tenofovir DF (TDF) is a nucleotide analog with potent antiviral activity in patients mono-infected with HBV and co-infected with HIV/HBV
HBV pol/RT resistance mutations have been identified following administration of oral anti-HBV agents (lamivudine, adefovir dipivoxil, entecavir, and telbivudine)
The rtA194T substitution was observed in two HIV/HBV co-infected patients1, however in a recent study the presence of this mutation did not result in reduced efficacy of TDF2
No amino acid substitutions associated with resistance to tenofovir were detected during the first 48 weeks of Studies 102 and 103
OBJECTIVES
To identify amino acid substitutions in the HBV pol/RT following 96 weeks of therapy with TDF 300 mg QD
To evaluate the effects of these substitutions on the clinical response to TDF mono-therapy
To determine whether these substitutions alter susceptibility to tenofovir using in vitro HBV replication assays and to evaluate the cross-resistance profi le of these substitutions
METHODS
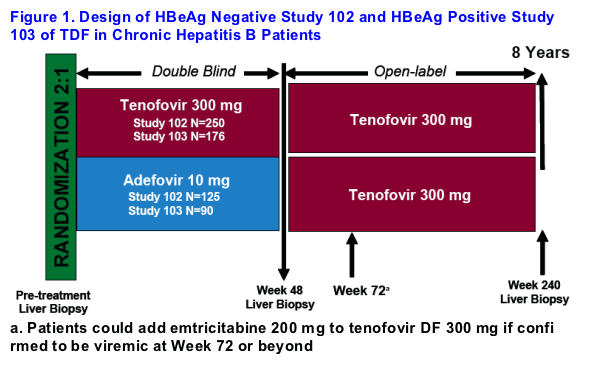
Patients were enrolled in one of two double-blind, randomized studies of TDF [GS-US-174-0102 (HBeAg-) or GS-US-174-0103 (HBeAg+)]
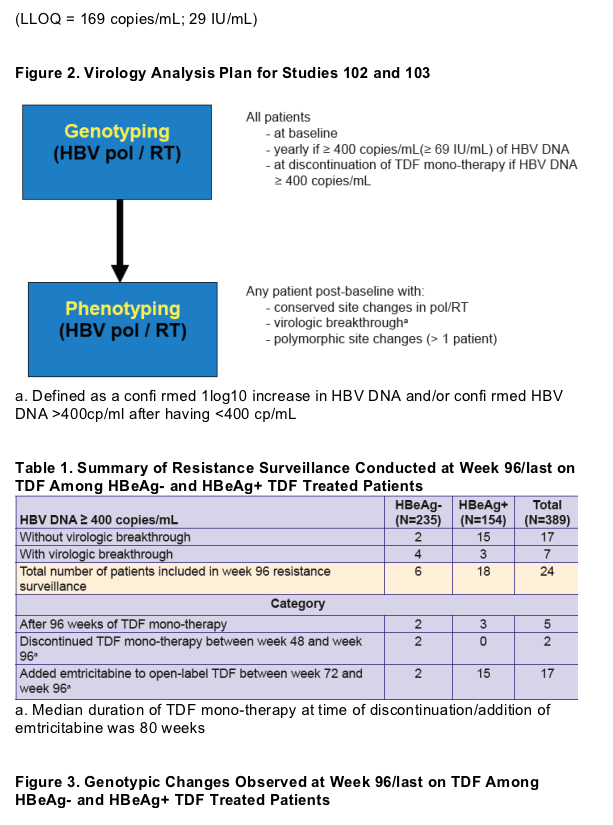
Population di-deoxy sequencing of serum HBV pol/RT
-- Covers AA 1-344 of pol/RT (AA 1-266 of HBsAg)
-- Able to detect AA substitutions present at ≥ 25% of viral quasi-species population
Phenotypic analyses were conducted in HepG2 cells transiently transfected with a pool of recombinant HBV plasmid DNA derived from patient serum HBV
Plasma HBV DNA levels were determined by Roche COBAS TaqMan assay (LLOQ = 169 copies/mL; 29 IU/mL)
REFERENCES
1. Sheldon et al. Antiviral Therapy, 10:727, 2005
2. Fung et al. AASLD 2008, Poster #880
|
| |
|
 |
 |
|
|