 |
 |
 |
| |
Kinetics Of HBsAg Decline During And Following Treatment of CHB: Early And Rapid HBsAg Decline During Peginterferon alfa-2a Is Predictive Of HBsAg Clearance
|
| |
| |
Reported by Jules Levin
59th Annual Meeting of the American Association for the Study of Liver Diseases, October 31 - November 4 2008, San Francisco, USA
M Brunetto,1 D Cavallone,1 F Moriconi,1 P Colombatto,1 G Moscato,2 AM Maina,1 F Oliveri,1 P Ciccorossi,1 B Coco,1 F Bonino3
1UO Epatologia, Azienda Ospedaliero Universitaria Pisana, Pisa, Cisanello, Italy; 2UO Laboratorio Centrale, Azianda Ospedaliero Universitaria Pisana, Pisa, Cisanello, Italy;
3Direzione Scientifica, Policlinico di Milano, IRCCS, Milano and UniversitÓ di Pisa, Milano, Italy
BACKGROUND
HBsAg clearance/seroconversion, spontaneous or treatment induced, is the hallmark of successful immunological control of HBV infection
Favorable clinical outcomes associated with HBsAg clearance following interferon therapy include decreased rates of cirrhosis and liver cancer, and an increase in life expectancy1-3
We demonstrated previously that (pegylated) interferon-based therapy is more effective than nucleos(t)ide analogs (NAs) in reducing serum HBsAg levels4,5
OBJECTIVE
In a single-center, prospective cohort study we evaluated whether the decline of serum HBsAg could be used to predict the long-term outcome [cure (HBsAg loss\anti-HBs seroconversion) and\or combined sustained response [HBV DNA <10,000 copies/mL and normal alanine aminotransferase (ALT)] after peginterferon alfa-2a therapy in clinical practice
AUTHOR SUMMARY
In this long-term observational study, only patients treated with peginterferon alfa-2a (monotherapy or in combination with NAs) achieved HBsAg clearance
Most patients who cleared HBsAg did so within 2 years after the end of peginterferon alfa-2a therapy having achieved a rapid decline of HBsAg (≥ 1 log in a 3-month interval) within the first 6 months of treatment
A more gradual progressive decline in HBsAg levels leading to eventual HBsAg clearance can also be observed following peginterferon alfa-2a therapy
AUTHOR CONCLUSION
Our study confirms that only patients treated with peginterferon alfa-2a (monotherapy or in combination with NAs) achieved HBsAg clearance. Two different patterns of HBsAg decline were observed in peginterferon alfa-2a-treated patients:
1. Rapid (> 1 log in a 3-month interval) and early (within the first 6 months
of treatment) decline that was associated with HBsAg clearance within 2 years post treatment and cure of chronic hepatitis B
2. Slow, progressive decline that continued after EOT and was associated with combined sustained response (normal ALT and HBV DNA < 104 copies/mL) and might eventually lead to HBsAg clearance in a longer follow up
Further studies are in progress to investigate the underlying mechanisms
METHODS
84 consecutive patients with chronic hepatitis B (12 HBeAg positive, 72 HBeAg negative) who received long-term treatment with NAs (lamivudine with or without adefovir) or 12-18 months' treatment with peginterferon alfa-2a (alone or in combination with lamivudine with or without adefovir) were studied
The patients were followed up for a median of 5 years (range 1_8 years)
HBsAg was measured annually using the Abbott Architect i2000 HBsAg assay and every 3 months if an HBsAg >1 log was found at the yearly control visit
HBV DNA (Amplicor Roche , dynamic range 400-200,000 copies/mL; samples with viremia higher than 200,000 copies/mL were retested after 1:100 dilution according to manufacturers' recommendations) and ALT [upper level of normal (ULN) 30/L] were tested at least every 3 months during treatment and post-treatment follow-up period
For NAs, on therapy, and for peginterferon alfa-2a plus NAs at the end of therapy (EOT), virological response was defined as undetectable HBV DNA (<400 copies/mL). For peginterferon alfa-2a, off therapy virological response was defined as the persistence of HBV DNA suppression (<10,000 copies/mL or <400 copies/mL) throughout the post-treatment period until the end of follow up (EOF)
Combined response was defined by the persistence of HBV DNA <10,000 copies/mL and normal ALT throughout the post-treatment follow-up period
RESULTS
Patient recruitment and enrolment
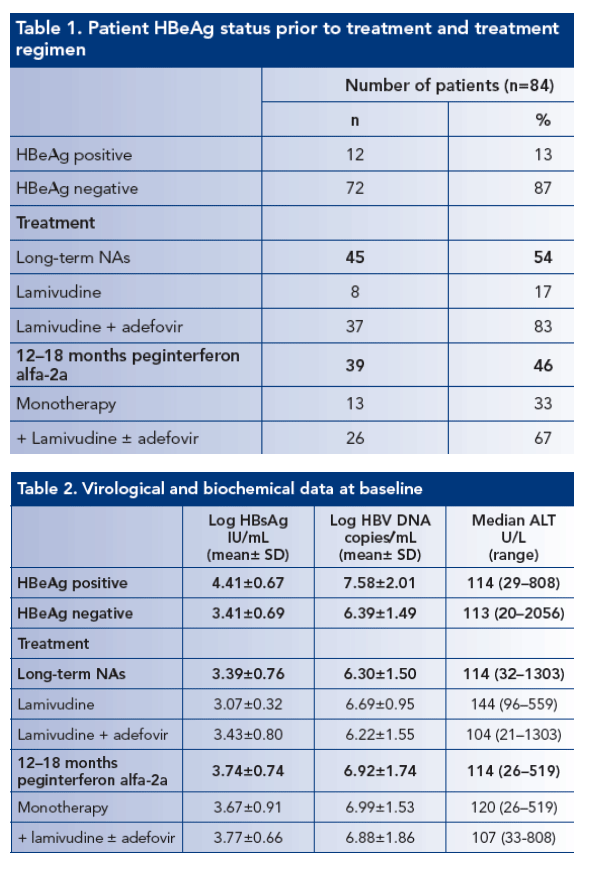
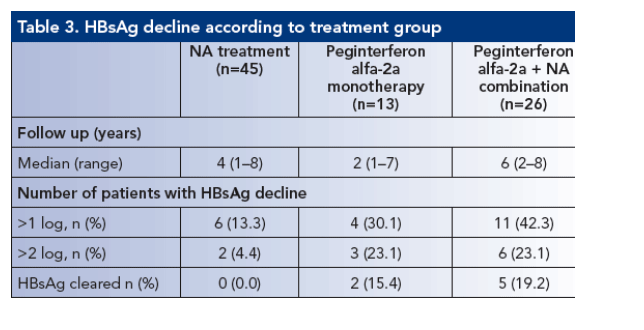
-- 84 consecutive patients were studied, the majority (87%) were HBeAg negative. Approximately one-half received treatment with peginterferon alfa-2a, either alone or in combination with NAs
-- Treatment schedules and baseline virological and biochemical features are shown in Tables 1 and 2
-- Genotype prevalence was: D in 73 patients, A in eight patients, F in two patients and C in one patient
The overall median follow up after starting treatment was 5 years (range: 1-8 years)
-- 4 (1-8 years) for those who received NAs
-- 2 (2-7 years) for those who received peginterferon alfa-2a alone
-- 6 (2-8 years) for those who received peginterferon alfa-2a plus NAs
Virological response
Virological response on therapy was present in 93.3% of NA-treated patients. The EOT/on-therapy response was present in 92.3% of peginterferon alfa-2a plus NA-treated patients, and was maintained at EOF in 12 patients (30.7%). Seven of the 39 peginterferon alfa-2a ▒ NA-treated patients (17.9%) had undetectable HBsAg during posttreatment follow up
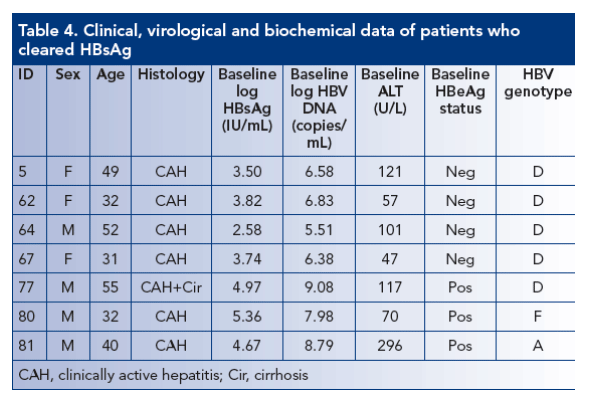
HBsAg decline from baseline
The degree of HBsAg decline according to treatment regimen is shown in Table 3
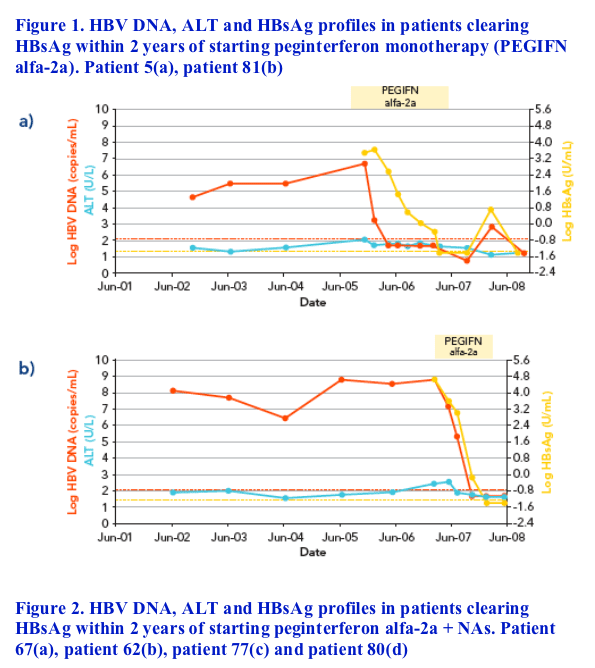
HBsAg clearance was observed in a total of seven patients - all of whom had received a peginterferon alfa-2a-containing regimen (two peginterferon alfa-2a alone, four peginterferon alfa-2a plus lamivudine and one peginterferon alfa-2a plus lamivudine plus adefovir). Baseline parameters of patients clearing HBsAg are shown in Table 4
In six of the seven patients, HBsAg decline was characterized by a rapid decline (> 1 log in a 3-month interval) that started at a mean time of 4.7▒1.8 months from the beginning of therapy, followed by subsequent HBsAg clearance within 2 years from starting treatment (Figures 1 and 2)
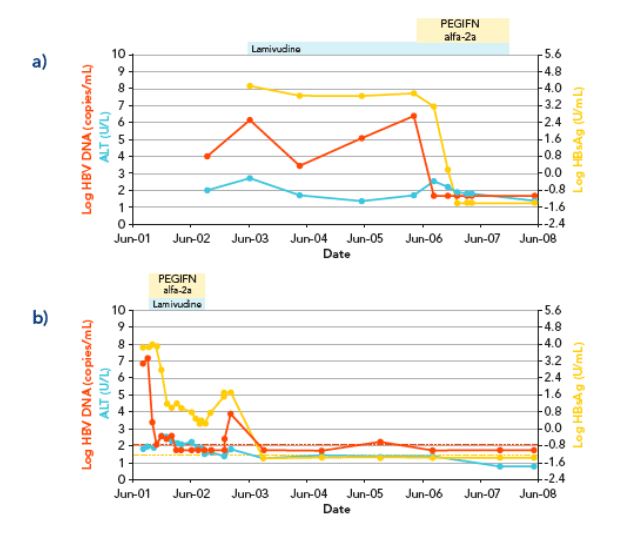
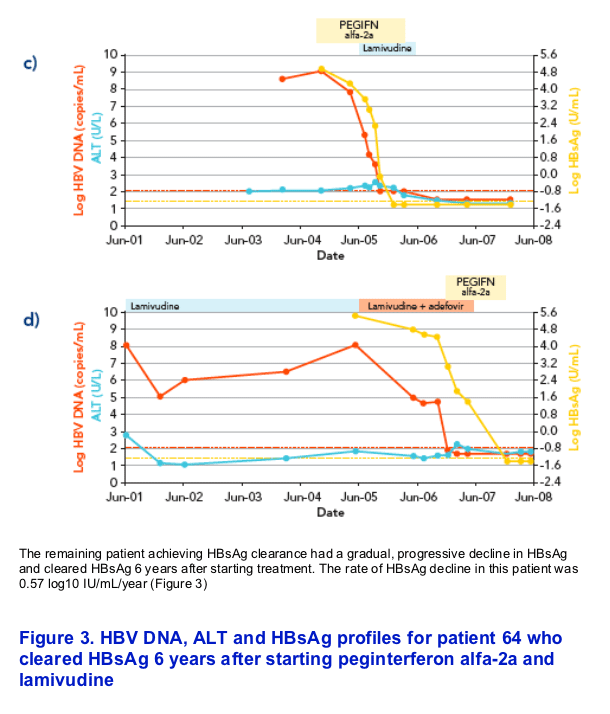
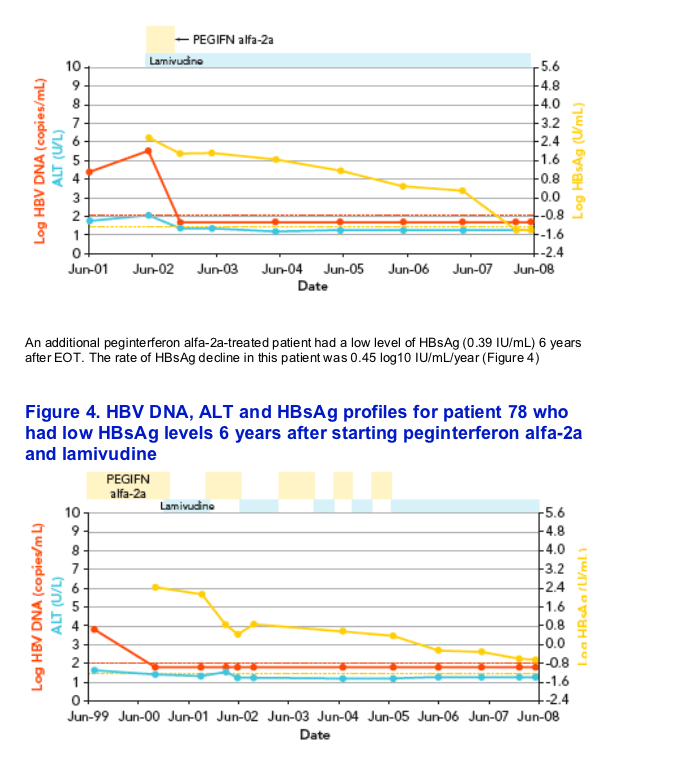
REFERENCES
1. Fattovich G et al. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP). Am J Gastroenterol
1998;93:896-900
2. Fattovich G et al. Long-term outcome of hepatitis B e antigen-positive patients with compensated cirrhosis treated with interferon alfa. European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology 1997;26(5):1338-42
3. Lin S-M et al. Interferon in HBeAg-positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol 2007;46:45-52
4. Brunetto M et al. Reduction in serum HBsAg level in patients with chronic
hepatitis B infected with genotype D induced by (pegylated) interferon alfa-2a
alone or in combination with nucleos(t)ide analogs: a long-term single centre cohort study. Hepatology 2007;46(Suppl):679A (Abstract 990)
5. Hou J et al. Efficacy and safety of peginterferon alfa-2a versus adefovir dipivoxil (ADV) in treating lamivudine resistant HBeAg positive CHB: an interim analysis of a prospective randomized study. AASLD 2008, P978
Disclosure Information
Editorial support for this poster was funded by F. Hoffmann-La Roche Ltd,
Basel, Switzerland
|
| |
|
 |
 |
|
|