| |
HBV DNA suppression induced by peginterferon alfa-2a (40KD) but not by lamivudine results in HBsAg clearance and seroconversion at 3 years off-treatment
|
| |
| |
Reported by Jules Levin
43rd EASL, April 2008, Milan Italy
Maurizia Brunetto1, Ferruccio Bonino2, Patrick Marcellin3, George K.K. Lau4, Patrizia Farci5, Cihan Yurdaydin6, Teerha Piratvisuth7, Stephanos Hadziyannis8, Zhi-Meng Lu9, Jian Wu10, Matei Popescu11
1UO Gastreonterologia ed Epatologia, Azienda Ospedaliera Universitaria Pisana, Pisa, Italy; 2Scientific Direction, Foundation IRCCS Policlinico of Milan and University of Pisa, Italy; 3Service d'Hepatologie, Hopital Beaujon, Clichy, France;
4Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong, China; 5Dipartimento di Scienze Mediche, Universita di Cagliari, Monserrato, Italy; 6Department of Gastroenterology, University of Ankara, Turkey;
7Department of Internal Medicine, Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Thailand; 8Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece; 9Department of Infectious Diseases, Ruijin Hospital, Shanghai, China; 10Roche, Dee Why, Australia; 11Roche, Basel, Switzerland
This research was partly funded by Roche, Basel, Switzerland
INTRODUCTION
· Hepatitis B e antigen (HBeAg)-negative chronic hepatitis B (CHB) represents a late phase in the course of HBV infection, characterized by progressive liver damage1
· Rates of sustained treatment response in HBeAg-negative patients are generally poor due to the high probability of relapse, particularly following nucleos(t)ide analogue therapy2-4
· Significantly more HBeAg-negative CHB patients treated with peginterferon alfa-2a for 48 weeks achieved HBV DNA 20,000 copies/mL and ALT normalisation at 24 weeks off-treatment than patients who received lamivudine in the same study5
· Furthermore, HBsAg clearance was seen in peginterferon alfa-2a-treated patients, but not in those who received lamivudine alone5
· 3 years after end of treatment HBsAg clearance had increased to 8% in those patients who had received peginterferon alfa-2a but had not occurred in those who had received lamivudine alone
· Here we examine the association of HBV DNA reduction at week 72 and the differences seen in HBsAg clearance rates between peginterferon alfa-2a-treated and lamivudine-treated patients
AUTHOR SUMMARY
· Patients with HBeAg-negative CHB treated with PEGASYS with or without LAM for 48 weeks had superior rates of response, including HBsAg clearance, by 3 years after end of treatment compared with patients treated with LAM monotherapy
· Substantial reduction in HBsAg level up to week 72 was seen only in patients who had reduced their HBV DNA 400 copies/mL following treatment with a PEGASYS-containing regimen, ie not in those who had HBV DNA suppression following LAM monotherapy
· All but one patient who subsequently cleared HBsAg after PEGASYS treatment had HBV DNA suppressed to 400 copies/mL at the end of treatment and retained suppressed levels of HBV DNA throughout follow-up. However, HBV DNA suppression appears to play only a first step to subsequent clearance of HBsAg
· Moreover, only patients who received PEGASYS cleared HBsAg by year 3 of follow-up:
- 8% of patients treated with PEGASYS cleared HBsAg versus none treated with LAM monotherapy
· HBsAg clearance following PEAGSYS treatment was seen across all major HBV genotypes
AUTHOR CONCLUSION
· In this study, only HBV DNA reduction with PEGASYS was associated with reduction and clearance of HBsAg; while LAM suppressed HBV DNA, it did not have any effect on HBsAg levels and did not result in subsequent clearance of HBsAg by 3 years after the end of treatment
· The ability of PEGASYS-based therapy to induce HBsAg clearance is likely due to its dual antiviral and immunomodulatory properties
· HBV DNA suppression appears to be a first step in subsequent HBsAg clearance but is not sufficient if induced by LAM only
· This ability of PEGASYS to induce HBsAg clearance - an outcome that is associated with improved survival - supports its use as a first-line treatment for patients with HBeAg-negative disease avoiding the need for long-term therapy and the associated risks of developing drug resistance in those patients who achieve and maintain their response
STUDY AIMS
· To determine the HBsAg clearance rates in patients treated with peginterferon alfa-2a (PEGASYS) with or without lamivudine (LAM) versus LAM in patients with HBeAg-negative CHB
· To investigate a potential relationship between HBV DNA suppression at week 72 and subsequent HBsAg clearance
METHODS
· In the initial study, 537 patients were randomised to receive 48 weeks treatment with PEGASYS (n=177), PEGASYS plus LAM (n=179) or LAM (n=181) and were then followed for 6 months (Figure 1)
- All patients were positive for HBsAg and negative for anti-HBs pre-treatment
· 116, 114 and 85 patients, respectively, entered an observation-only follow-up study
· The numbers of patients with normalised ALT levels, suppression of HBV DNA (≦20,000 copies/mL, ≦10,000 or ≦400 copies/mL) and HBsAg clearance were recorded at 1, 2 and 3 years after the end of treatment
· HBV DNA was measured using the COBAS AMPLICOR HBV MONITOR (Roche Diagnostics, Branchburg, NJ, USA)
· HBsAg and anti-HBs antibodies were measured using the ABBOTT ARCHITECT assay (Abbott Laboratories, Abbott Park, Ill, USA)
RESULTS
Overall results 3 years after end of treatment
· At 3 years off-treatment, the percentage of patients with normal ALT or HBV DNA levels ≦10,000 copies/mL was significantly higher for patients treated with PEGASYS monotherapy (31% and 28%, respectively) than with LAM monotherapy (18% and 15%; P=0.034 and P=0.041, respectively) (Table 1)
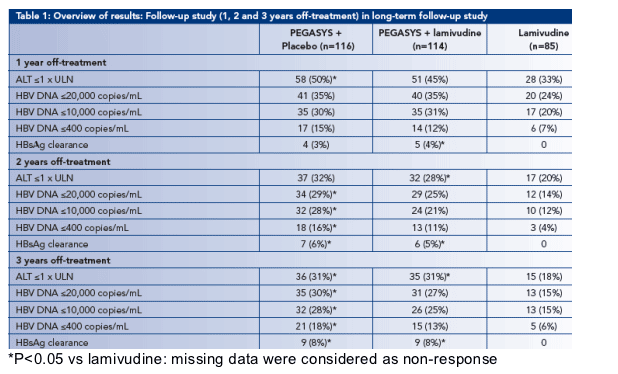
HBsAg response at 3 years off-treatment
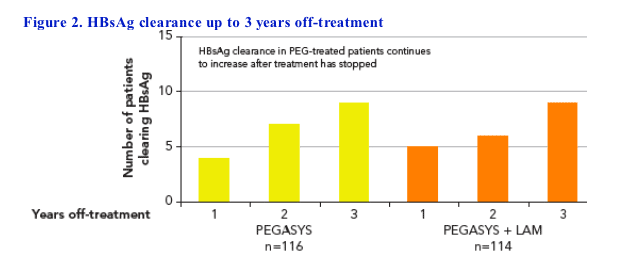
· Off-treatment benefits of peginterferon alfa-2a treatment are reflected in the continual increase in the proportion of patients who achieved HBsAg clearance and/or seroconversion during the off-treatment follow-up period (Figure 2):
- The proportion of all patients treated with PEGASYS who cleared HBsAg increased from 3-4% at 6 months off-treatment to 8% at 3 years off-treatment (n=18)
- 44% (n=8) of patients who cleared HBsAg also developed anti-HBs at 3 years
· No patient treated with lamivudine alone cleared HBsAg at 3 years (P=0.009 for HBsAg clearance: PEGASYS ± LAM vs LAM)
Stratification of HBsAg response according to HBV DNA response at end of treatment and during follow-up: All PEGASYStreated patients (PEGASYS and PEGASYS + LAM)
Of 181 PEGASYS-treated patients with HBV DNA ≦400 copies/mL at end of treatment, 17 (9.4%) cleared HBsAg at 3 years post-treatment versus 1 of the 49 patients with HBV DNA >400 copies/mL
Most patients who cleared HBsAg at 3 years had undetectable HBV DNA levels from end of treatment through to 3-year follow-up
No patient who cleared HBsAg had HBV DNA >14,000 copies/mL at 1 year off treatment
35% of patients with an HBV DNA level ≦400 copies/mL 1 year after end of treatment had sustained HBsAg clearance 3 years after end of treatment (Figure 3)
Despite the fact that a high percentage of patients who were treated with LAM alone achieved suppression of HBV DNA ≦400 copies/mL at end of treatment (83%), none of them cleared HBsAg during the 3-year treatment-free follow-up phase
Relationship between HBsAg level decline and HBV DNA suppression by PEGASYS or LAM
There was an overall association between HBsAg level reduction and HBV DNA level from pre-treatment to end of treatment (week 72) (Figure 4)
- The mean decline in HBsAg level from pre-treatment to week 48 was greater for patients with HBV DNA ≦400 copies/mL (-1.077 log10 IU/mL) versus patients with HBV DNA >400 copies/mL (-0.263 IU/mL; P<0.001)
However, analysis according to treatment group showed that this was only in patients who received PEGASYS, alone or combined with LAM
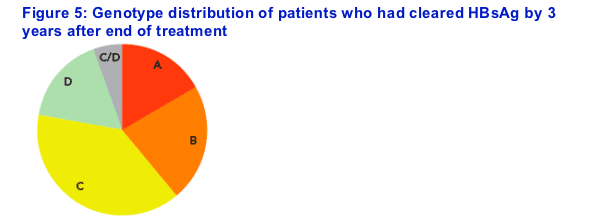
Stratification of HBsAg response by genotype
Loss of HBsAg was observed across all major genotypes (Figure 5)
REFERENCES
1. Hadziyannis SJ et al. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2001;34:617-24.
2. Hadziyannis SJ et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med 2005;352:2673-81.
3. Manesis EK, Hadziyannis SJ. Interferon a treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastroenterology
2001;121:101-9.
4. Hadziyannis SJ et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology
2006;131(6):1743-51.
5. Marcellin P et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic
hepatitis B. N Engl J Med 2004;351:1206-17.
|
|
| |
| |
|
|
|