| |
Efficacy and Safety of Entecavir in Patients With Chronic Hepatitis B and Advanced Hepatic Fibrosis or Cirrhosis
|
| |
| |
The American Journal of Gastroenterology
Nov 2008
Eugene Schiff, M.D., M.A.C.P., F.R.C.P., M.A.C.G. 1 , Halis Simsek, M.D. 2 , William M. Lee, M.D. 3 , You-Chen Chao, M.D. 4 , Hoel Sette, Jr., M.D. 5 , Harry L.A. Janssen, M.D., Ph.D. 6 , Steven-Huy Han, M.D. 7 , Zachary Goodman, M.D., Ph.D. 8 , Joanna Yang, Ph.D. 9 , Helena Brett-Smith, M.D. 9 , and Ricardo Tamez, M.D. 10
1 University of Miami, Miami, Florida ; 2 Department of Internal Medicine, Hacettepe University Medical School, Ankara, Turkey ; 3 University of Texas Southwestern Medical Center, Dallas, Texas ; 4 Tri-Service General Hospital, Taipei, Taiwan ; 5 Hospital das Clinicas, Sao Paulo, Brazil ; 6 Erasmus MC University Medical Center, Rotterdam, The Netherlands ; 7 David Geffen School of Medicine at UCLA, Los Angeles, California ; 8 Armed Forces Institute of Pathology, Washington, DC ; 9 Bristol-Myers Squibb Company, Research & Development, Wallingford, Connecticut ; and 10 Bristol-Myers Squibb Company, Research & Development, Princeton, New Jersey
Reprint requests and correspondence: Eugene Schiff, M.D., Room 1101 Jackson Medical Towers East, 1500 NW 12th Avenue, Miami, Florida 33136.
Presented, in part, at the 41st Annual Meeting of the European Association for the Study of the Liver, April 26-30, 2006, and 42nd Annual Meeting of the European Association for the Study of the Liver, April 11-16, 2007.
"STUDY HIGHLIGHTS
What Is Current Knowledge
--Hepatitis B virus (HBV) may result in severe fibrosis/cirrhosis.
--Patients with severe fibrosis/cirrhosis are at the greatest risk of morbidity and mortality as a result of HBV infection and, thus, gain the most from effective therapies.
--Entecavir improves histologic, virologic, and biochemical outcomes in both nucleos(t)ide-naive and lamivudine-resistant patients with advanced liver fibrosis.
What Is New Here
--Entecavir is as effective in patients with advanced fibrosis/cirrhosis as in the overall population."
ABSTRACT
OBJECTIVE: The efficacy and safety of entecavir in patients with chronic hepatitis B and advanced liver fibrosis/cirrhosis was assessed from three large, randomized, multicenter, phase III studies.
PATIENTS AND METHODS: These studies enrolled patients (≥16 yr) with chronic hepatitis B, elevated alanine aminotransferase (ALT) levels, and compensated liver disease. Two trials enrolled nucleos(t)ide-naive patients randomized to at least 48 wk of treatment with entecavir 0.5 mg/day or lamivudine 100 mg/day. The third trial randomized lamivudine-refractory patients to 48 wk of entecavir 1 mg/day or lamivudine 100 mg/day. In this post hoc descriptive analysis, the efficacy and safety in patients with advanced liver fibrosis/cirrhosis (Ishak fibrosis stages 4-6) were examined for consistency with those seen in the overall study populations.
RESULTS: Of the 1,633 treated patients, 245 had advanced liver fibrosis/cirrhosis (120 entecavir and 125 lamivudine). Among entecavir-treated patients with advanced liver fibrosis, improvement in Ishak fibrosis was observed in 57% of nucleos(t)ide-naive hepatitis B e antigen (HBeAg)-positive patients, 59% of nucleos(t)ide-naive HBeAg-negative patients, and 43% of lamivudine-refractory HBeAg-positive patients versus 49%, 53%, and 33% of lamivudine-treated patients with advanced liver fibrosis. The overall trends in other histologic, virologic, biochemical, and serologic outcomes in entecavir- versus lamivudine-treated patients with advanced liver fibrosis/cirrhosis were consistent with those observed in the overall study populations in each trial. The treatment was well tolerated.
CONCLUSION: These data confirm that the performance of entecavir relative to that of lamivudine in patients with advanced liver fibrosis/cirrhosis was consistent with the relationship observed in the overall treated population.
INTRODUCTION
It is estimated that 400 million people are chronically infected with hepatitis B virus (HBV) (1), and that about one-third (30%) of reported cases of cirrhosis and more than one-half (53%) of reported cases of hepatocellular carcinoma (HCC) are caused by chronic hepatitis B (CHB) infection (2). The risk of death in patients with CHB increases with the extent of hepatic impairment. The 5-yr mortality rate is 16% in patients with compensated cirrhosis, but increases to 65-86% in individuals with decompensated cirrhosis (3-5). Thus, an effective treatment for CHB is urgently needed, particularly for patients with advanced liver fibrosis and/or cirrhosis.
Liaw et al. (6) have shown that continuous treatment with lamivudine delays clinical progression of patients with CHB and advanced fibrosis or cirrhosis (defined as Ishak fibrosis stages 4-6). After a median of 32 months of follow-up, the incidence of disease progression (defined by a composite end point of hepatic decompensation, HCC, spontaneous bacterial peritonitis, bleeding gastroesophageal varices, and liver-related death) was significantly lower in patients treated with lamivudine than with placebo (hazard ratio 0.45, P= 0.001) (6). Although initially effective in a high proportion of patients with CHB, lamivudine is associated with a high incidence of resistance (7); thus, the suitability of lamivudine as a first-line agent for CHB has been questioned (8, 9). Nucleos(t)ide substitutions resulting in the evolution of drug resistance have been observed with all other nucleos(t)ide analogs. Cumulative probabilities of genotypic resistance to adefovir of 0%, 3%, 11%, 19%, and 30%, at 1, 2, 3, 4, and 5 yr, respectively, were reported in a phase III trial in hepatitis B e antigen (HBeAg(-)) patients (10). Telbivudine has demonstrated lower rates of resistance than lamivudine, but recently, the reported virologic breakthrough due to resistance substitutions was 21.6% and 8.6% in HBeAg-positive and HBeAg-negative patients after 2 yr of treatment, respectively (11). Entecavir (Baraclude; Bristol-Myers Squibb, Princeton, New Jersey) has demonstrated the lowest reported rates of resistance (1.2-1.7%) after 3-5 yr (12, 13).
Entecavir is a potent guanosine analog that has shown to reduce the incidence of HCC and increase survival in an animal model of chronic HBV infection (14). In nucleos(t)ide-naive patients with HBeAg-positive and HBeAg-negative CHB, 48 wk of treatment with entecavir 0.5 mg/day demonstrated a superior virologic, histologic, and biochemical efficacy than lamivudine (15, 16). A continued treatment of HBeAg-positive patients beyond 48 wk resulted in increasing proportions of patients achieving and maintaining virologic, biochemical, and serologic end points through 4 yr of therapy with minimal resistance (17, 18). In lamivudine-refractory patients with CHB who participated in a large randomized, multicenter phase III trial, switching to entecavir 1 mg/day was significantly more effective than continuing lamivudine treatment (19).
The three large, randomized, double-blind, multinational phase III trials that evaluated entecavir each included a subpopulation of patients with advanced liver fibrosis/cirrhosis as defined in the trial by Liaw et al. (6) The aim of this analysis was to assess the efficacy and safety of entecavir in the subgroup of patients with advanced liver fibrosis/cirrhosis in these studies.
MATERIALS AND METHODS
The complete study design and patient selection criteria for the three trials have been published elsewhere (15, 16, 19).
Study Design
In all three studies, eligible patients were randomized to receive a minimum of 48 wk of a double-blind treatment with either entecavir (Baraclude) or lamivudine (Epivir-HBV, GlaxoSmithKline, Brentford, UK) (15, 16, 19). Nucleos(t)ide-naive patients received entecavir at a dosage of 0.5 mg once daily (15, 16). Lamivudine-refractory patients were randomized to switch to entecavir at a dosage of 1 mg once daily or to continue lamivudine (18). The dosage of lamivudine in all three trials was 100 mg once daily (15, 16, 19).
Patients
Patients eligible for the three trials were adults aged ≥16 yr, with a serum HBV DNA level ≥3 mEq/mL (if HBeAg-positive) or ≥0.7 mEq/mL (if HBeAg-negative) by the Quantiplex (Bayer Diagnostics, Emeryville, CA) branched-chain DNA assay, serum alanine aminotransferase (ALT) levels between 1.3 and 10 times the upper limit of normal (ULN), and compensated liver disease. Compensated liver disease was defined as a total serum bilirubin level ≦2.5 mg/dL, serum albumin level ≥3.0 g/dL, prolongation of the normal prothrombin time by ≦3 s (or international normalized ratio ≦1.5), and no history of variceal bleeding or hepatic encephalopathy (i.e., Child-Pugh class A). The patients were also required to have biopsy-proven evidence of chronic hepatitis, a serum alpha-fetoprotein level ≦100 ng/mL, and infection by hepatitis C or D viruses or human immunodeficiency virus.
Efficacy Assessments
The key efficacy end points for this analysis were the same as in the initial analyses of the overall study populations: histologic improvement, defined as a ≥2-point decrease in the Knodell necroinflammatory score, with no worsening of the Knodell fibrosis score at week 48 of study treatment. Biopsies were scheduled to be performed at the baseline (unless one had been performed within 1 yr before randomization) and at week 48. Liver biopsy specimens were evaluated by a central independent histopathologist who was unaware of individual patient's treatment assignment, the biopsy sequence, and clinical outcome. For the purposes of this analysis, patients with advanced liver fibrosis/cirrhosis include those who had a baseline Ishak fibrosis score of 4, 5, or 6.
The secondary efficacy end points included the proportion of patients with improvement in Ishak fibrosis score at week 48 (≥1-point decrease from the baseline), the proportion of patients with undetectable HBV DNA (<300 copies/mL, by Roche COBAS Amplicor PCR assay, Roche Diagnostics, Pleasanton, CA), and the proportion of patients with normalization of serum ALT levels (i.e., ≦1.0 x ULN). The emergence of resistance to entecavir (i.e., emergence of amino acid substitutions associated with resistance to entecavir and development of virologic breakthrough due to genotypic resistance to entecavir) was also evaluated. Genotyping was performed on paired baseline and on-treatment samples from all patients who experienced a virologic breakthrough (confirmed 1 log or more increase in HBV DNA from the nadir). In addition, genotyping was performed on paired samples from all patients having HBV DNA ≥300 copies/mL at week 48.
Safety Assessments
Safety assessments included adverse events, serious adverse events, discontinuations due to clinical or laboratory-determined adverse events, and deaths. On-treatment ALT flares (defined as serum ALT levels >2 _ baseline and >10 x ULN) and off-treatment flares (defined as serum ALT levels >2 _ reference and >10 x ULN) were also reported.
Statistical Analyses
All patients who received at least one dose of the study medication and had an adequate baseline biopsy specimen and a Knodell necroinflammatory score ≥2 were included in the primary efficacy analyses. The present analysis is a descriptive subgroup analysis of week 48 responses in patients with biopsy-proven advanced liver fibrosis/cirrhosis (Ishak fibrosis scores of 4-6) at baseline who received study, treatment and had a week 48 liver biopsy.
Tabulations by treatment group are presented for each of the efficacy and safety variables. Continuous variables are summarized with the mean values. Binary variables are summarized by counts and percentages. A 2-sided Fisher's exact test procedure was used to compare entecavir with lamivudine on the efficacy end points.
RESULTS
A total of 1,633 patients were randomized to the treatment in the three trials and received at least one dose of the study medication (Table 1). Of the total population, 245 (15%) patients had biopsy-proven evidence of advanced liver fibrosis/cirrhosis; 120 of whom received entecavir, and 125 of whom received lamivudine. The baseline characteristics of nucleos(t)ide-naive patients and lamivudine-refractory patients included in this analysis are presented in Tables 1 and 2. The characteristics of patients with advanced liver fibrosis/cirrhosis were similar to those of the overall populations in all three studies, although patients with advanced liver fibrosis typically were older than those without advanced liver fibrosis in each study. Also, slightly higher mean ALT levels were observed in naive, but not lamivudine-refractory, patients with advanced liver fibrosis as compared with the overall population.
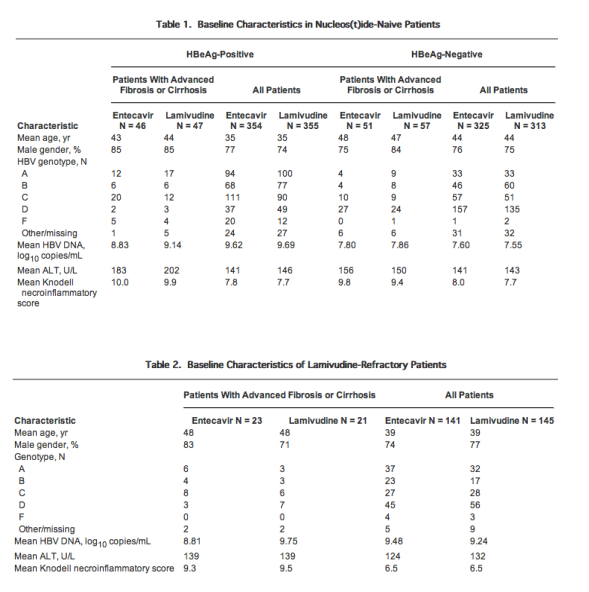
Efficacy
A greater number of entecavir- versus lamivudine-treated patients with advanced liver fibrosis/cirrhosis achieved histologic improvement (defined as a ≥2-point improvement in the Knodell necroinflammatory score and no worsening of fibrosis) at week 48 among nucleos(t)ide-naive HBeAg-positive (80%vs 64%), nucleos(t)ide-naive HBeAg-negative (75%vs 60%), and lamivudine-refractory HBeAg-positive patients (57%vs 29%) (Tables 3 and 4). The rates of histologic improvement were consistent between the subgroup with advanced liver fibrosis and the overall population treated (Tables 3 and Table 4).
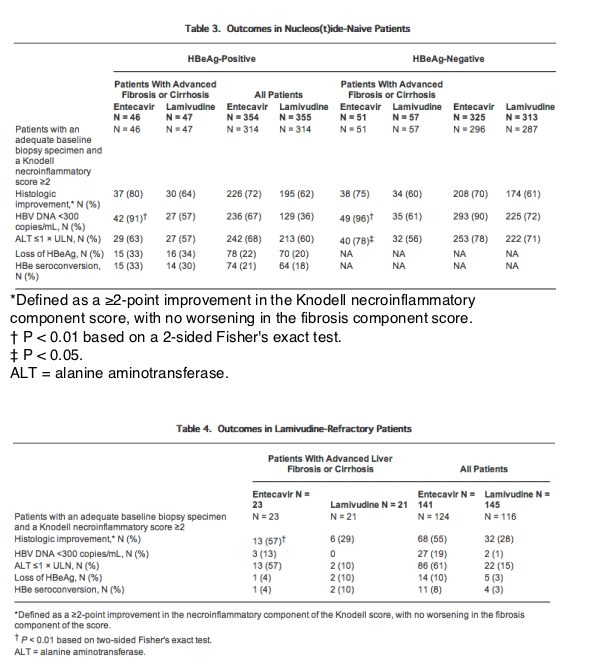
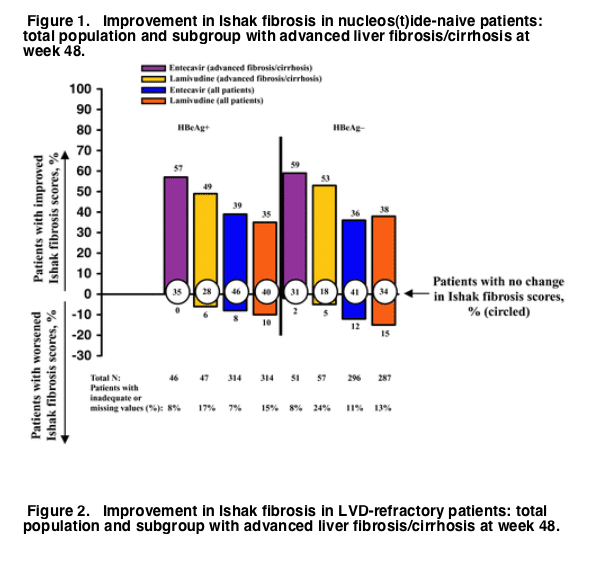
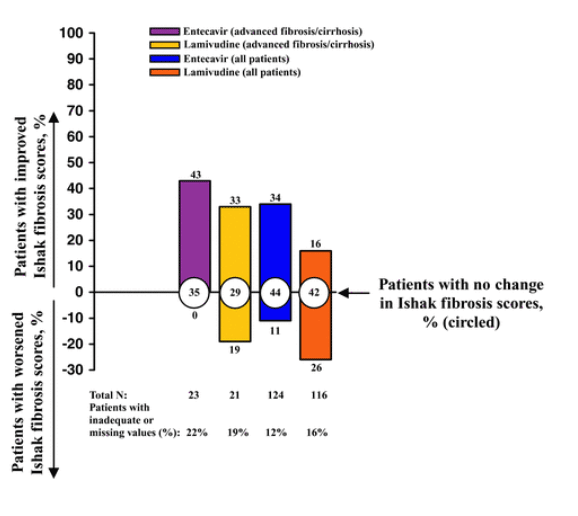
Among patients with advanced liver fibrosis/cirrhosis treated with entecavir, improvement in Ishak fibrosis at week 48 was observed in 57% of nucleos(t)ide-naive HBeAg-positive patients, 59% of nucleos(t)ide-naive HBeAg-negative patients, and 43% of lamivudine-refractory HBeAg-positive patients, as compared with 49%, 53%, and 33% of patients treated with lamivudine (Figs. 1 and 2). These values are higher than those seen for the total population treated with entecavir, in which improvement in Ishak fibrosis at week 48 was observed in 39% of nucleos(t)ide-naive HBeAg-positive patients, 36% of nucleos(t)ide-naive HBeAg-negative patients, and 34% of lamivudine-refractory HBeAg-positive patients, as compared with 35%, 38%, and 16% of patients treated with lamivudine (Figs. 1 and 2). Worsening of Ishak fibrosis was not observed in entecavir-treated HBeAg-positive patients with advanced liver fibrosis/cirrhosis, and was observed in only 2% of nucleos(t)ide-naive HBeAg-negative patients with advanced liver fibrosis/cirrhosis.
Among nucleos(t)ide-naive HBeAg-positive and HBeAg-negative patients treated with entecavir, a higher proportion of patients with advanced liver fibrosis/cirrhosis achieved HBV DNA <300 copies/mL by PCR (91% and 96%, respectively), as compared with lamivudine-treated patients (57% and 61%, respectively), which was consistent with the proportions of patients observed in the overall population (Table 3). Also consistent with the overall population, a higher proportion of lamivudine-refractory HBeAg-positive patients with advanced liver fibrosis treated with entecavir achieved HBV DNA <300 copies as compared with lamivudine-treated patients (13%vs 0%, respectively) (Table 4).
The proportion of patients with normalization of serum ALT levels among patients with advanced liver fibrosis/cirrhosis was higher for entecavir- versus lamivudine-treated patients (63%vs 57% for HBeAg-positive patients, 78%vs 56% for HBeAg-negative patients, and 57%vs 10% for lamivudine-refractory patients) and consistent with that observed in the overall population (Tables 3 and 4).
Although small numbers of HBeAg-positive patients with advanced liver fibrosis/cirrhosis achieved HBeAg loss or HBe seroconversion, the proportions were consistent with those observed in the overall populations (Tables 3 and 4).
No entecavir-treated patients with advanced liver fibrosis/cirrhosis had the emergence of amino acid substitutions associated with the resistance to entecavir or virologic breakthrough due to genotypic resistance to entecavir.
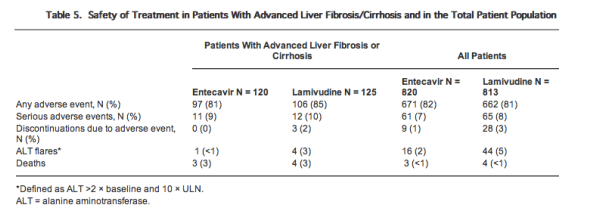
Safety
The frequency of on-treatment adverse events was comparable among the groups with advanced liver fibrosis/cirrhosis and with those reported in the overall study population (Table 5). Most frequent adverse events were similar to those described in the original studies. No entecavir-treated patients with advanced-liver fibrosis/cirrhosis discontinued the therapy due to adverse events. Three (2%) lamivudine-treated patients with advanced liver fibrosis/cirrhosis discontinued the study therapy due to adverse events. On-treatment ALT flares occurred in 1 entecavir-treated patient and in 4 lamivudine-treated patients with advanced liver fibrosis/cirrhosis. These rates were lower than those reported in the overall population, particularly in the lamivudine-refractory population treated with lamivudine. All deaths in the overall study population (3 in the entecavir group and 4 in the lamivudine group) occurred in patients with advanced liver fibrosis/cirrhosis. No deaths were considered related to study medication. Of the entecavir-treated patients, 1 died of gastrointestinal hemorrhage secondary to splenic lymphoma, 1 died of hepatocellular carcinoma, and 1 died of multiorgan failure secondary to diabetes and cirrhosis. Of the lamivudine-treated patients, 1 died of septic shock secondary to acute cholecystitis, 1 died of sudden dyspnea, 1 died of hepatic failure approximately 20 wk following treatment discontinuation due to an ALF flare, and 1 died of an undetermined cause.
DISCUSSION
Patients with advanced liver fibrosis/cirrhosis are at an increased risk of developing complications due to progression of CHB. This analysis demonstrates the efficacy of entecavir in patients with advanced liver fibrosis/cirrhosis, and that the overall benefits of entecavir versus lamivudine were consistent with those observed in the overall populations enrolled in three randomized, multicenter phase III clinical trials (15, 16, 19).
Improvements in Ishak fibrosis scores were more common among entecavir-treated patients with advanced liver fibrosis than in lamivudine-treated patients; conversely, fewer entecavir- than lamivudine-treated patients experienced progression of fibrosis during 48 wk of antiviral treatment. Also, among entecavir-treated patients, improvements in Ishak fibrosis scores were observed in a higher proportion of nucleos(t)ide-naive patients (57-59%) and lamivudine-refractory patients (43%) with advanced liver fibrosis/cirrhosis at the baseline than in the overall populations (34-39%).
These results from a small subset of the total population (15%) are likely to reflect the higher potential for improvement in patients with more advanced fibrosis at the baseline. However, high proportions of patients with advanced liver fibrosis also achieved HBV DNA <300 copies/mL and HBeAg seroconversion, providing confirmatory evidence for the efficacy of entecavir in patients with advanced liver fibrosis/cirrhosis.
In a hallmark study in patients with advanced liver disease, Liaw et al. demonstrated that continuous treatment with lamivudine delayed progression of liver disease (6). Although this study failed to provide information on histologic improvement, it showed that the development of antiviral resistance reduced the benefit obtained with lamivudine therapy. In this analysis of patients with advanced liver fibrosis/cirrhosis, treatment with entecavir resulted in suppression of HBV DNA below the limit of detection (300 copies/mL) in 91% and 96% of nucleos(t)ide-naive HBeAg-positive and HBeAg-negative patients, respectively. None of the patients with advanced liver fibrosis experienced the emergence of substitutions associated with entecavir resistance. These results are in line with the current American Association for the Study of Liver Diseases treatment guidelines, which recommended selecting a potent nucleos(t)ide analog with the lowest rate of genotypic resistance (20).
There is limited data on the efficacy of other treatments on patients with advanced liver disease. Recently, Buster et al., reported results on 70 HBeAg-positive patients with advanced fibrosis and 169 patients without it who were treated with either peginterferon alpha-2b alone or in combination with lamivudine (21). Virologic response and improvement in liver fibrosis were more common in patients with advanced fibrosis (25% and 66%) versus patients without advanced fibrosis (12% and 26%, P < 0.02 and P < 0.001, respectively) (21). It should be noted that in this study, HBV genotype A was more prevalent among patients with advanced fibrosis, and this may have contributed to the improved responses seen in patients with advanced fibrosis.
In the present study, the treatment was safe and well tolerated in patients with advanced liver fibrosis/cirrhosis, and both entecavir and lamivudine exhibited similar safety profiles. In particular, ALT flares occurred less frequently in entecavir-treated patients than in lamivudine-treated patients.
In conclusion, the efficacy of entecavir relative to that of lamivudine in patients with advanced liver fibrosis/cirrhosis was consistent with the relationship observed in the overall treated population in three large, randomized, multicenter phase III trials. Entecavir provided a higher observed virologic, histologic, and biochemical efficacy than lamivudine in the subgroup of patients with advanced liver fibrosis/cirrhosis. These data confirm that the efficacy of entecavir is consistent across a broad spectrum of patients with CHB. Furthermore, because patients with advanced liver fibrosis/cirrhosis are most likely to benefit from potent antiviral treatment, entecavir may be a good option for the first-line treatment in these patients.
|
|
| |
| |
|
|
|