 |
 |
 |
| |
ATRIPLA: The 48-Week Efficacy and Safety of Switching to Fixed-Dose Efavirenz/Emtricitabine/Tenofovir DF in HIV-1-Infected Patients Receiving HAART
|
| |
| |
Reported by Jules Levin
ICAAC/IDSA Oct 27, 2008 Wash DC
E DeJesus,1 A Pozniak,2 J Gallant,3 J Arribas,4 Y Zhou,5 A Cheng,5 and J Enejosa5
1Orlando Immunology Center, FL; 2Chelsea & Westminster Hosp, London, UK; 3Johns Hopkins Univ, Baltimore, MD;
4Univ Hosp La Paz, Madrid, Spain; 5Gilead Sciences, Inc., Foster City, CA, USA
BACKGROUND/METHODS
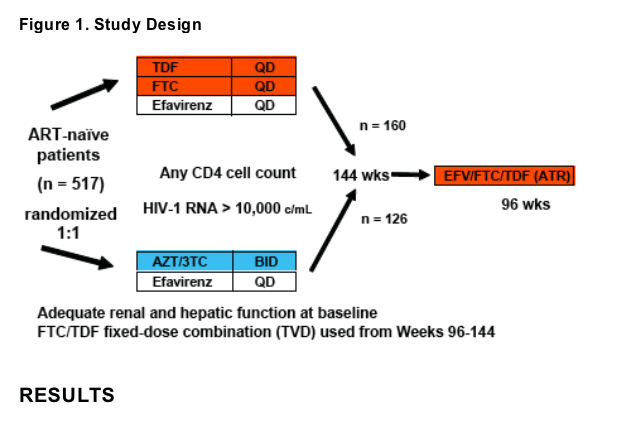
Study 934 is a 144-week randomized trial comparing the safety and efficacy of emtricitabine/tenofovir DF (TVD) or the individual components versus lamivudine/zidovudine (CBV) both in combination with efavirenz (EFV) in treatment-na´ve patients
After completing 144 weeks, patients in both arms were given the option to switch their antiretroviral regimen to the fixed-dose combination efavirenz/emtricitabine/tenofovir DF (ATR) once daily taken on an empty stomach, preferably at bedtime
Results after 48 weeks of follow-up post-switch are presented
AUTHOR CONCLUSIONS
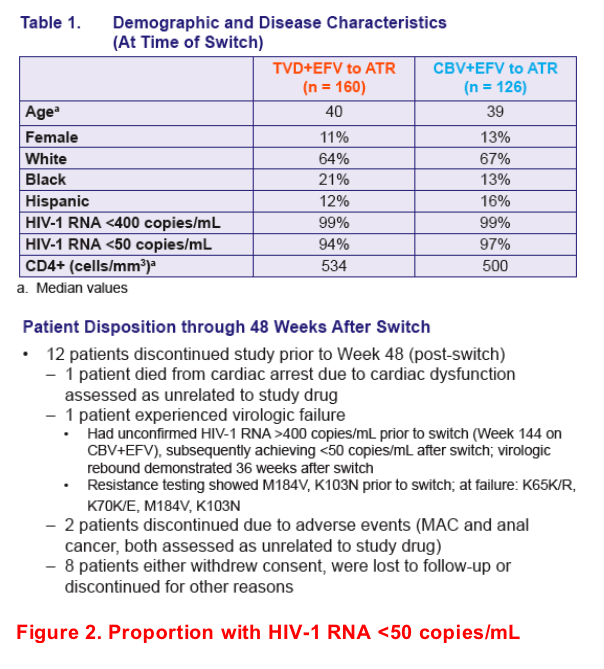
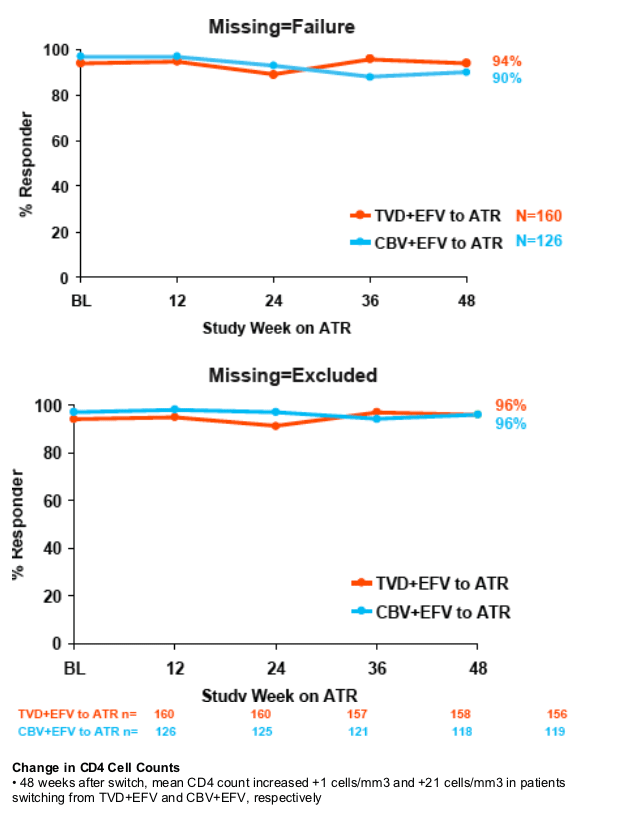
In patients receiving HAART for 144 weeks, virologic suppression was maintained 48 weeks after switching from TVD+EFV or CBV+EFV to a single tablet once-daily regimen of EFV/FTC/TDF (ATR)
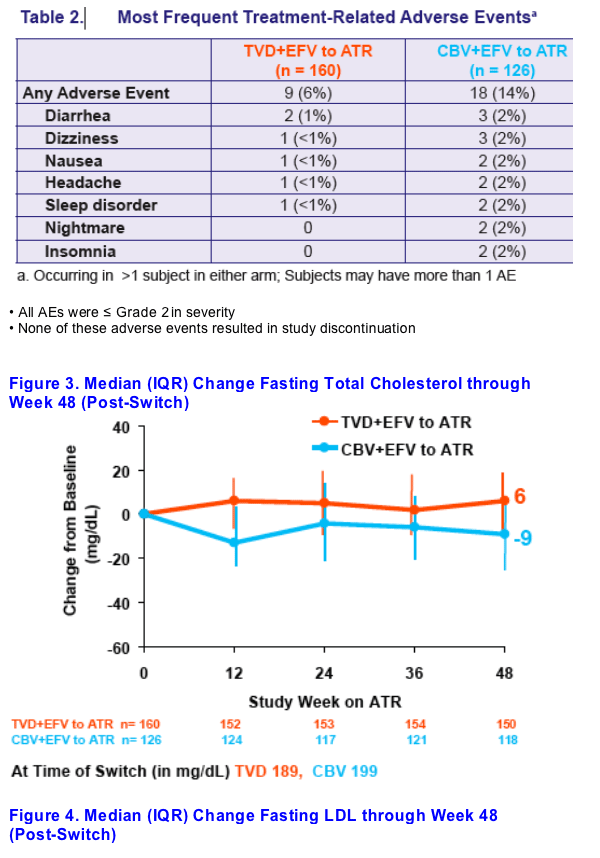
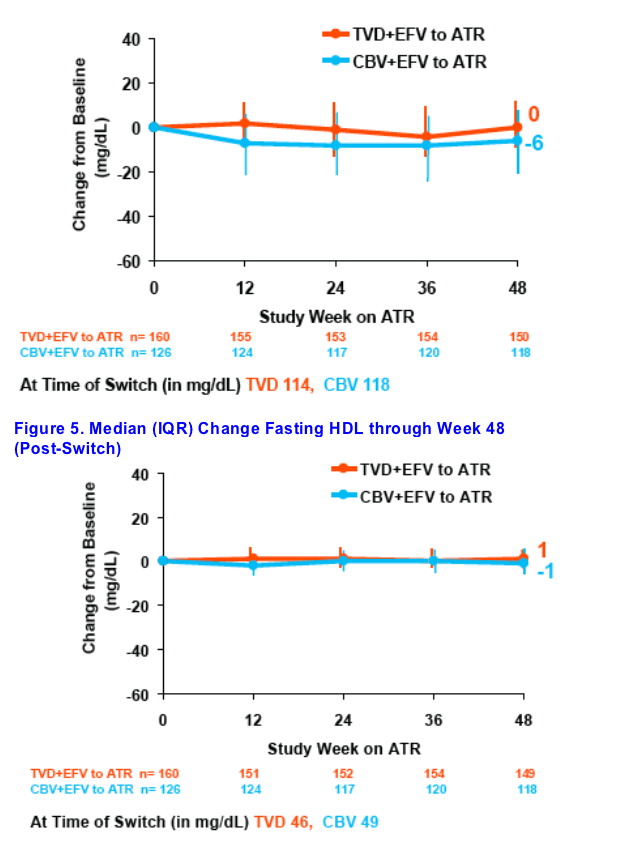
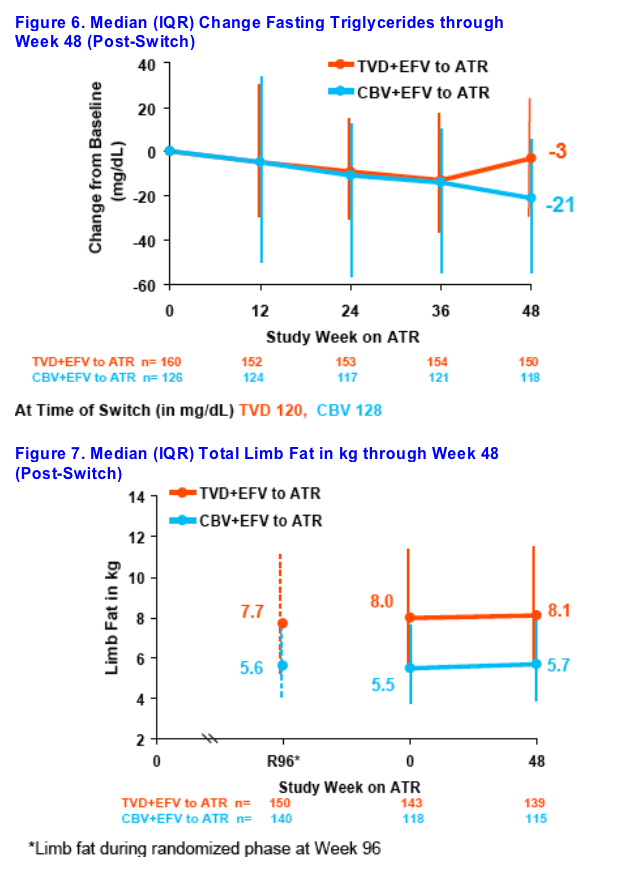
Decreases in fasting triglycerides and fasting cholesterol were seen 48 weeks after switching from CBV+EFV to ATR
Limb fat prior to switch was signifi cantly lower in CBV+EFV recipients, and no relevant changes were seen 48 weeks after switching from CBV+EFV to ATR
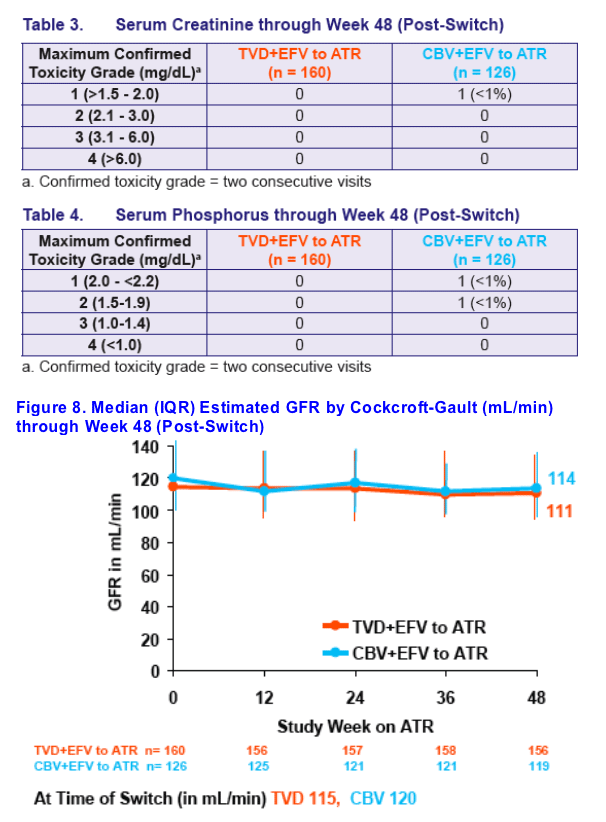
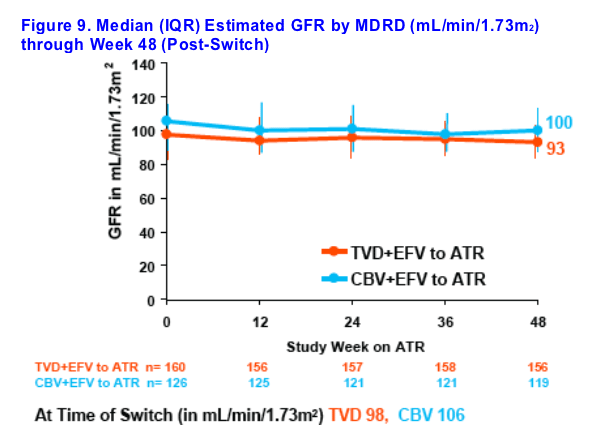
Renal function remained stable through 48 weeks post-switch
|
| |
|
 |
 |
|
|