 |
 |
 |
| |
Three Years Efficacy and Safety of Tenofovir Disoproxil Fumarate (TDF) in Asians with HBeAg-Positive and HBeAg-Negative Chronic Hepatitis B
|
| |
| |
Reported by Jules Levin
AASLD Nov 3 2009 Boston
S Lee1, E J Heathcote2, W Sievert3, H Trinh4, K Kaita5, Z Younossi6, J George7, M L Shiffman8, P Marcellin9, J Sorbel10, J Anderson10, E Mondou10, J Quinn10, F Rousseau10
1University of Calgary, Alberta, Canada; 2Toronto Western Hospital, University of Toronto, Ontario, Canada; 3Monash University, Melbourne, VIC, Australia; 4San Jose Gastroenterology, San Jose, CA, USA; 5University of Manitoba Health Science Center, Winnipeg
MB, Canada; 6Inova Fairfax Hospital, Falls Church, VA, USA; 7Storr Liver Unit, Westmead Hospital and University of Sydney, Westmead, NSW Australia; 8Virginia Commonwealth University Medical Center, Richmond VA, USA;
9Hopital Beaujon, Clichy France; 10Gilead Sciences, Durham NC, USA
INTRODUCTION
Tenofovir DF has demonstrated durable effi cacy and safety in 2 pivotal studies in chronic hepatitis B through 144 weeks (3 years) of treatment.
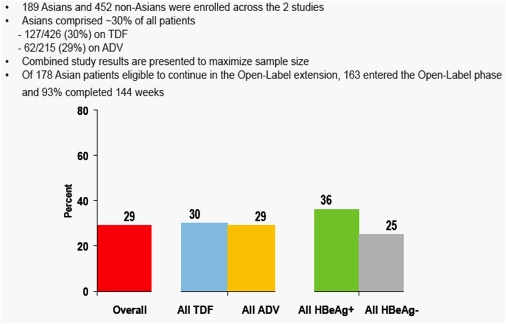
Asian patients comprised a substantial subset of the participants in these studies
Evaluation of efficacy and safety in Asian patients was considered important given the prevalence of HBV infection in this population
OBJECTIVE
To evaluate the effi cacy and safety of tenofovir DF among Asian patients with chronic hepatitis B participating in tenofovir DF pivotal studies GS-US-174-0102 (HBeAg-) and GS-US-174-0103 (HBeAg+)
METHODS
Patients were randomized 2:1 to double-blind tenofovir DF (TDF) 300 mg or adefovir dipivoxil (ADV) 10 mg once daily for 48 weeks
Open-label tenofovir DF commenced at week 48 for those patients completing the double-blind phase
Virologic (HBV DNA < 400 copies/mL [69 IU/mL]), biochemical, and serologic response were prospectively evaluated
HBV DNA and safety laboratory parameters* were performed every 4 weeks in year 1, every 8 weeks in year 2, and every 12 weeks in year 3 with annual resistance surveillance (*creatinine clearance calculated by Cockcroft-Gault
with actual body weight)
Asian ethnicity was determined by self-report as recorded on the case report form
Statistical methods are described in Posters 481 and 483
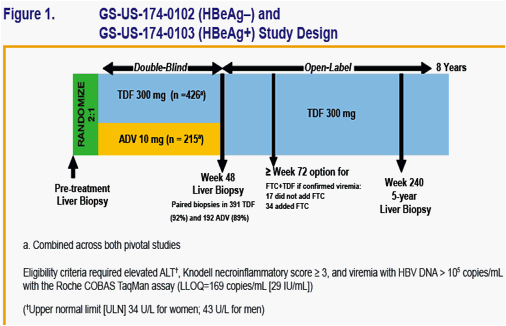
Figure 2. Asian Patients Participating in Pivotal Studies
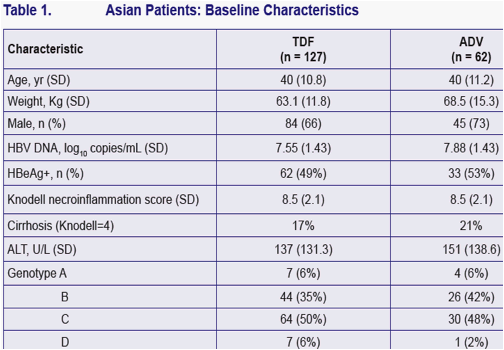
Values are means for continuous variables. ALT ULN= 34 U/L for women; 43 U/L for men
Figure 3. Number of Patients Remaining on Studies
Figure 4. Percentage of Patients with HBV DNA < 400 copies/mL (69 IU/mL) (LTE-TDF)
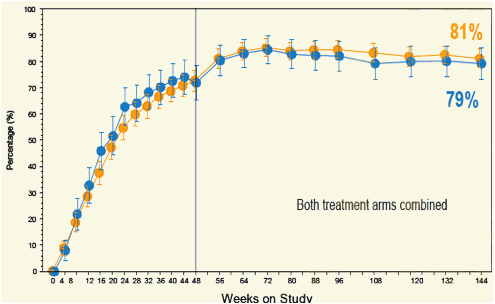
Error bars are 95% CIs.
ITT long-term evaluation with FTC+TDF considered as failure
In the Open-Label extension population with FTC+TDF considered as failure 83% of non-Asian and 87% of Asian patients had HBV DNA < 400 copies/mL (69 IU/mL) at Week 144
Figure 5. Percentage of Patients with HBV DNA < 400 copies/mL (69 IU/mL) (On-Treatment Analysis)

Error bars are 95% CIs
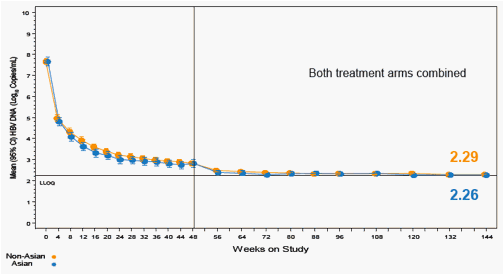
Figure 6. Mean HBV DNA Over Time (log10 copies/mL)
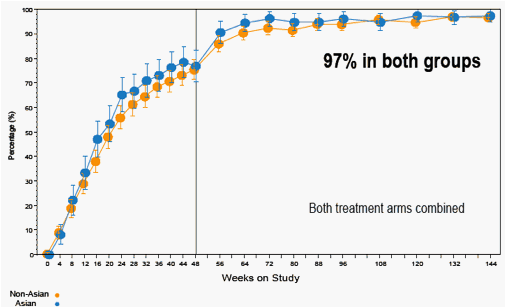
Figure 7. Percentage of Patients with Normal ALT (On-Treatment Analysis)
Error bars are 95% CIs.
ALT ULN= 34 U/L for women; 43 U/L for men
Figure 8. Mean ALT Over Time
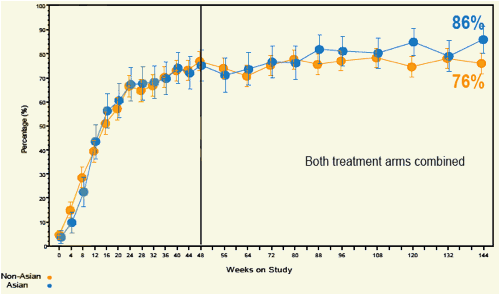
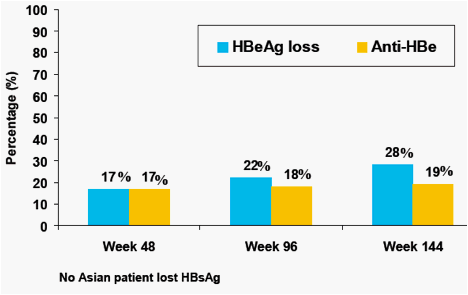
Figure 9. Serologic Response Among Asian Patients (On-Treatment Analysis)
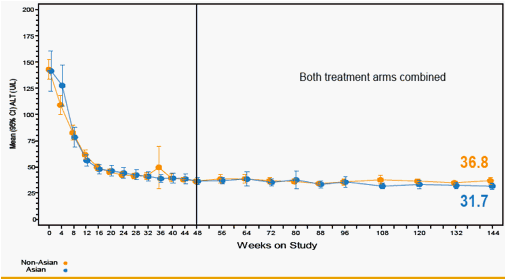
Table 2. Safety and Tolerability in Patients on TDF for 144 Weeks
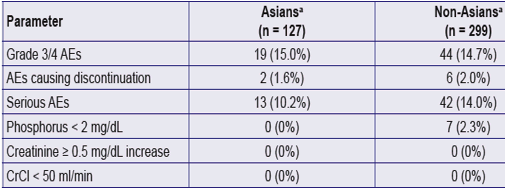
a. Asians and Non-Asians originally randomized to TDF (n=426; 127 Asians; 299 non-Asians)
One Asian patient died of poorly differentiated nasopharyngeal carcinoma, and 6/189 (3.2%) had fractures (none pathologic)
Table 3. Grade 3/4 Laboratory Values in Patients on TDF for 144 Weeks
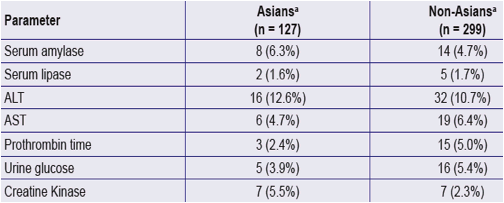
a. Asians and Non-Asians originally randomized to TDF (n=426; 127 Asians; 299 non-Asians)
Note: Includes Grade 3/4 laboratory parameters occurring in > 1 Asian patient
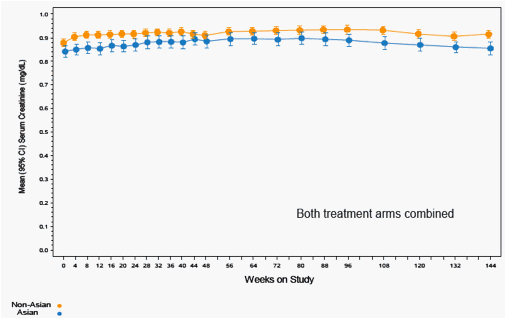
Figure 10. Serum Creatinine Over Time
TDF Resistance Surveillance
Comprehensive Week 144 resistance surveillance is presented in Poster 480
Across both pivotal studies 4 Asian patients had HBV DNA > 400 copies/mL (> 69 IU/mL) at Week 144
- 1 had no change from baseline in HBV pol/RT
- 2 had polymorphic site change
- 1 could not be genotyped
|
| |
|
 |
 |
|
|