 |
 |
 |
| |
HBsAg Decline in Patients Treated With PEGASYS and its Association with Post-treatment Response in HBeAg-Positive Chronic Hepatitis B
|
| |
| |
Reported by Jules Levin
Presented at the 19th Conference of the Asia Pacific Association for the Study of the Liver, 13-16 February 2009, Hong Kong, China
Lau GKK,1 Marcellin P,2 Brunetto M,3 Piratvisuth T,4 Kapprell H-P,5 Batrla R,6 Popescu M6
1Department of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong, China. 2Service d'Hepatologie and Centre de Recherches Biologiques Bichat Beaujon (Inserm CRB3),
University of Paris, Clichy, France. 3UO Epatologia, Azienda Ospedaliero Universitaria Pisana, Pisa, Cisanello, Italy. 4NKC Institute of Gastroenterology and Hepatology, Department of Internal Medicine,
Songklanagarind Hospital, Prince of Songkla University, Hat Yai, Thailand. 5Abbott GmbH & Co, Wiesbaden-Delkenheim, Germany. 6Hoffmann-La Roche Ltd, Basel, Switzerland
Author Summary
PEGASYS treatment either alone or in combination with lamivudine results in a considerable decline in HBsAg level from baseline. Low levels of HBsAg at a number of timepoints during therapy were associated with high rates of response after completion of therapy - even as much as 1-year post treatment
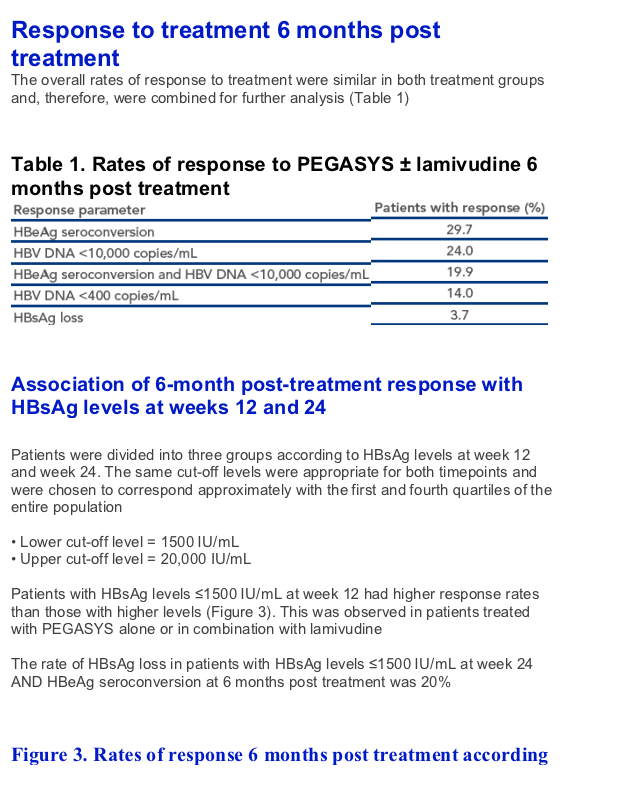
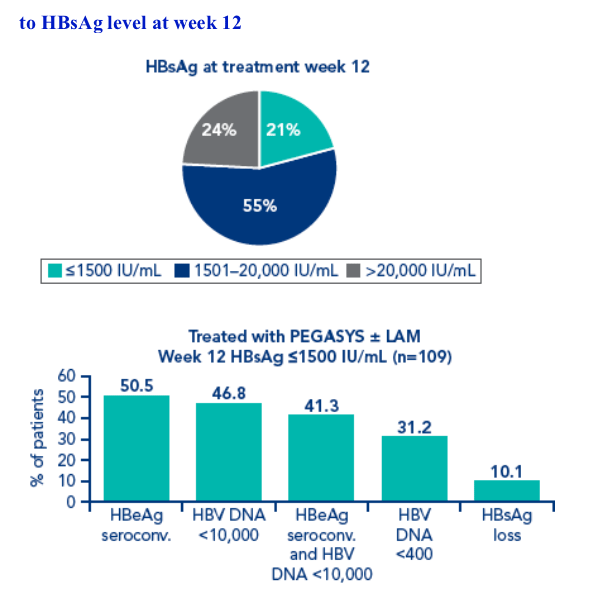
At week 12, an HBeAg seroconversion rate of 51% was achieved 6 months post treatment by the 21% of patients who had week 12 HBsAg levels ≦1500 IU/mL
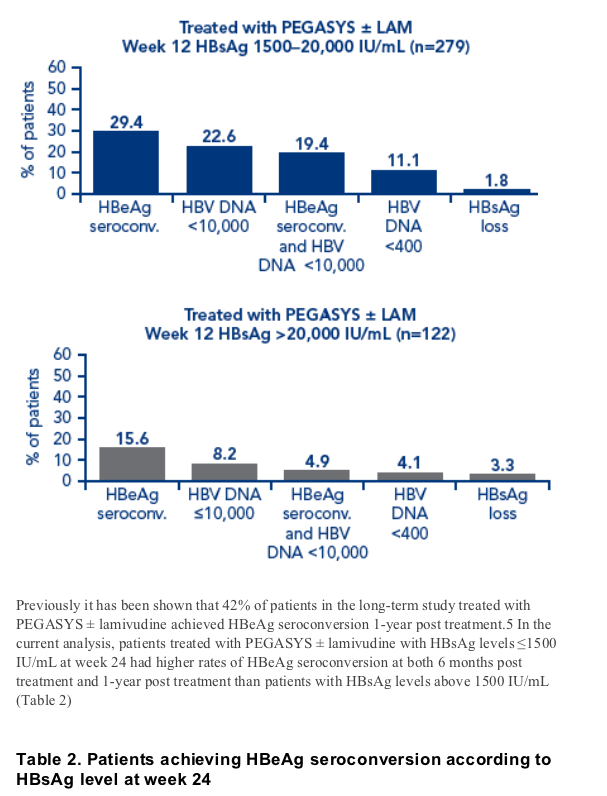
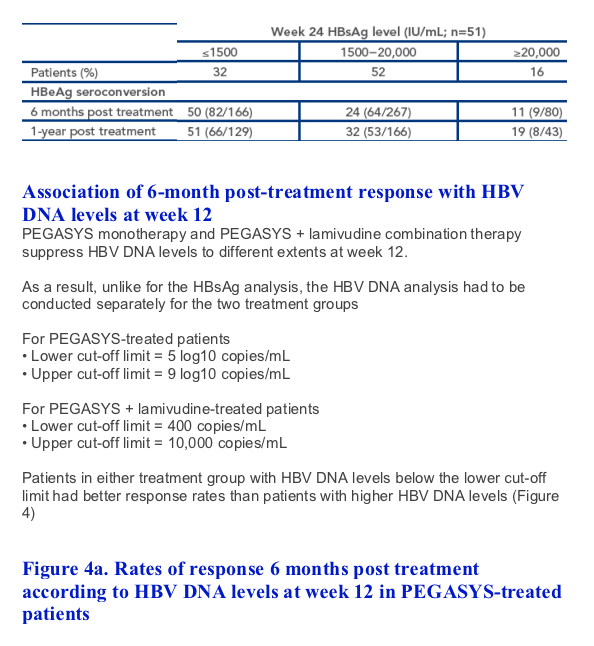
A favorable response to therapy could be predicted in more patients using HBsAg levels at week 24. An HBeAg seroconversion rate of 50% was achieved by the 32% of patients with HBsAg levels below 1500 IU/mL at week 24
Importantly, an HBeAg response rate of 51% was achieved 1-year post treatment in patients with HBsAg levels <1500 IU/mL at week 24 highlighting that this is a long-term durable benefit of PEGASYS-based therapy
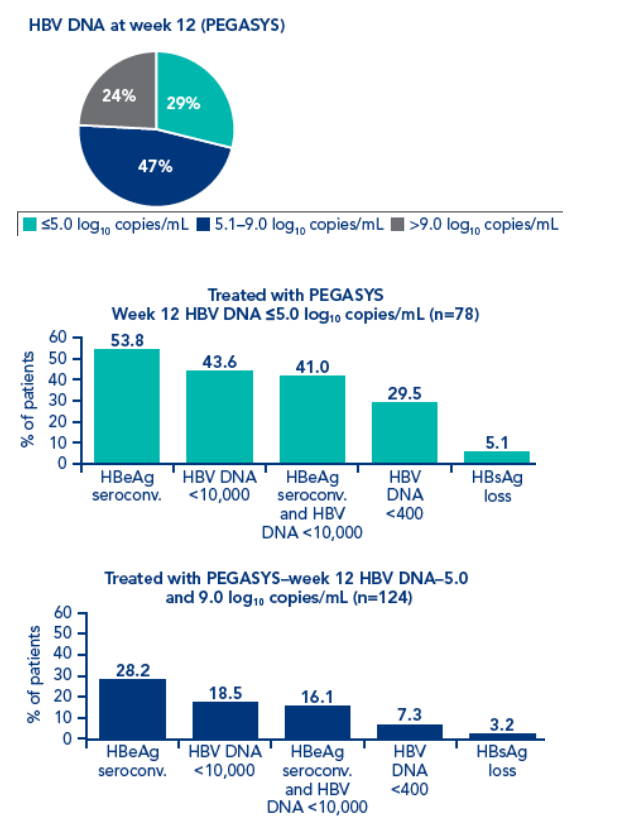
Although HBV DNA levels at week 12 were associated with post treatment response, different cut-off levels had to be defined for each treatment regimen
Rates of HBsAg at 6 months and one year treatment in patients with low HBsAg levels at weeks 12 and 24 were higher than in patients with low HBV DNA levels at week 12
Author Conclusions
HBsAg levels during treatment were associated with response 6 months post treatment in patients with HBeAg-positive disease. Response could be predicted as early as week 12, but could be predicted with more confidence at week 24. Importantly, HBsAg levels at week 24 could predict favorable response to PEGASYS therapy in the 6 months following treatment and 1 year after completion of therapy. HBsAg during treatment can, therefore, be used as an early predictor of durable post-treatment response to PEGASYS-based therapy in individual patients
Background
Patients with HBeAg-positive disease who achieve a sustained post-treatment response to interferon-based therapy have an increasing chance of developing HBsAg clearance during long-term follow up.1 HBsAg clearance is the ultimate goal of therapy in patients with chronic hepatitis B (CHB) and is associated with long-term favorable outcomes
In a large-scale study, we showed that a 48-week course of PEGASYS with or without lamivudine resulted in HBsAg clearance and seroconversion in patients with HBeAg-positive CHB.2 This response was not observed with lamivudine alone
It has been suggested that on-therapy reductions in serum HBsAg levels or HBV DNA levels may act as useful markers of response to therapy in patients with CHB3,4
Advances in quantification of HBsAg levels have allowed us to assess the relationship between HBsAg decline during treatment with response to therapy post treatment
Objective
We investigated the association between on-treatment HBsAg decline and sustained response in patients treated with PEGASYS with or without lamivudine. We also assessed the relationship between on-treatment HBV DNA levels and response to therapy
Methods
The initial large-scale study in HBeAg-positive patients consisted of three treatment arms - PEGASYS (180 μg/week, n=271), PEGASYS + lamivudine (100 mg/day, n=271) and lamivudine alone (n=271).2 Patients received treatment for 48 weeks and were then followed up for an additional 24 weeks. The endpoint of the study was 6 months post treatment (72 weeks)
In the current analysis, only data from the two PEGASYS arms was available Quantitative HBsAg levels in serum was measured retrospectively pretreatment and at weeks 12, 24 and 48 of treatment using the Abbott Architect HBsAg assay. HBsAg level was also determined 6 months post treatment
The association between response 6 months post treatment and HBsAg level at week 12 or week 24 and HBV DNA level at week 12 was determined. Response was defined as:
· HBeAg seroconversion
· HBV DNA <10,000 copies/mL
· Combined response: HBeAg seroconversion and HBV DNA <10,000 copies/mL
· HBV DNA <400 copies/mL
· HBsAg loss
The association between response 1-year post treatment and HBsAg levels during treatment was also determined
Results
HBsAg level decline from baseline
Baseline levels of HBsAg were similar in both treatment groups
· Mean HBsAg level in PEGASYS alone group = 4.09 log10 IU/mL
· Mean HBsAg in PEGASYS + lamivudine group = 4.16 log10 IU/mL
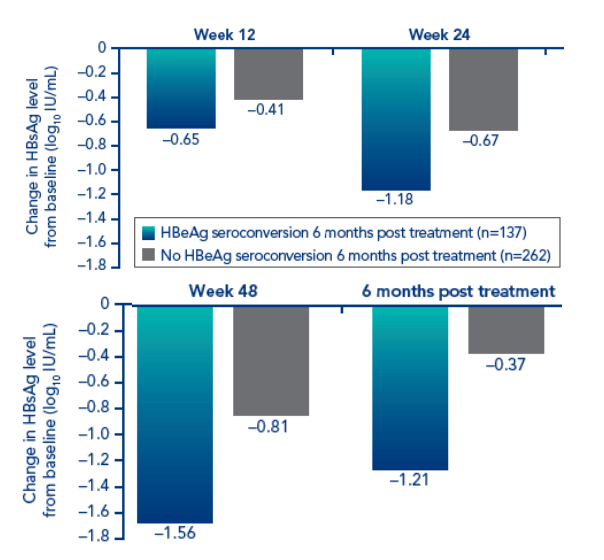
Decrease in HBsAg level during the treatment period was slightly more pronounced in the combination therapy group than in the PEGASYS alone group (Figure 1).
There was some rebound in HBsAg level between end of treatment at week 48 and 6 months post treatment. This was greater in the PEGASYS + lamivudine group (from -1.1 log10 IU/mL to -0.6 log10 IU/mL) than in the PEGASYS monotherapy group (from -0.8 log10 IU/mL to -0.5 log10 IU/mL)
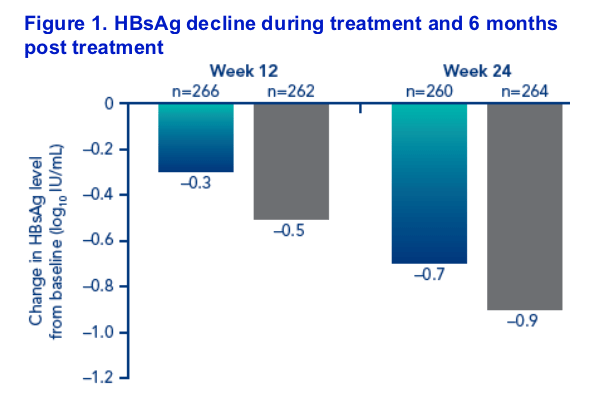
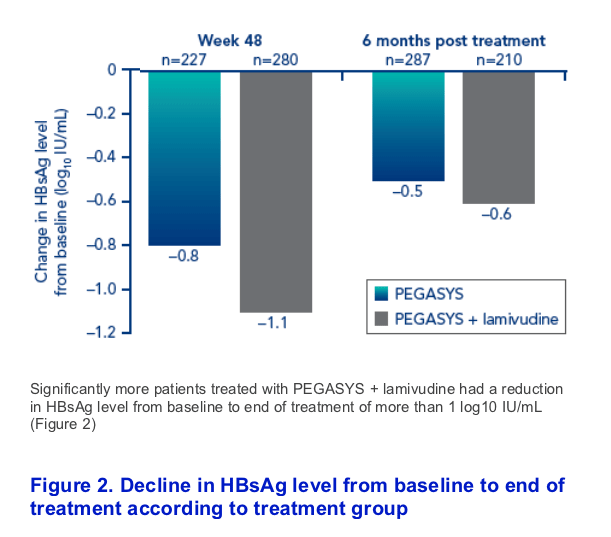
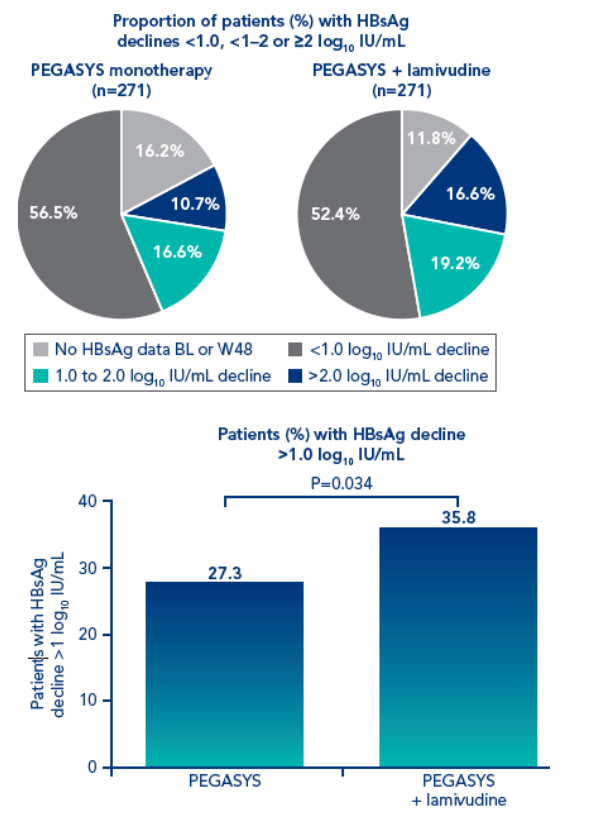
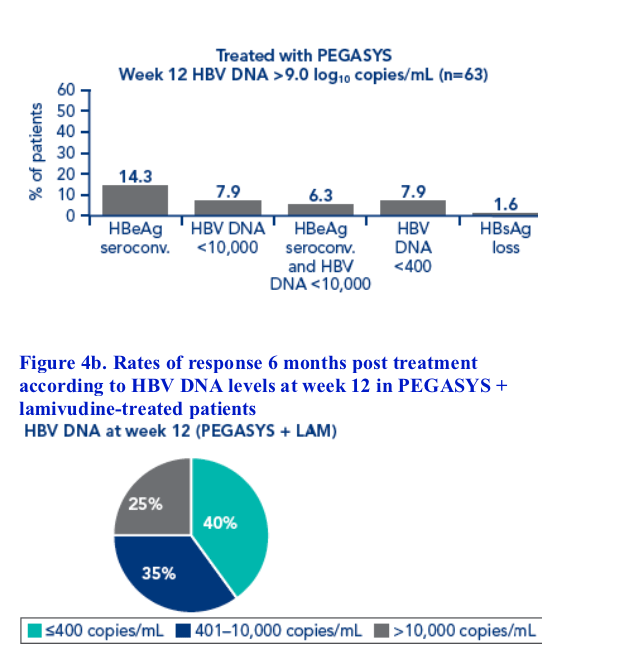
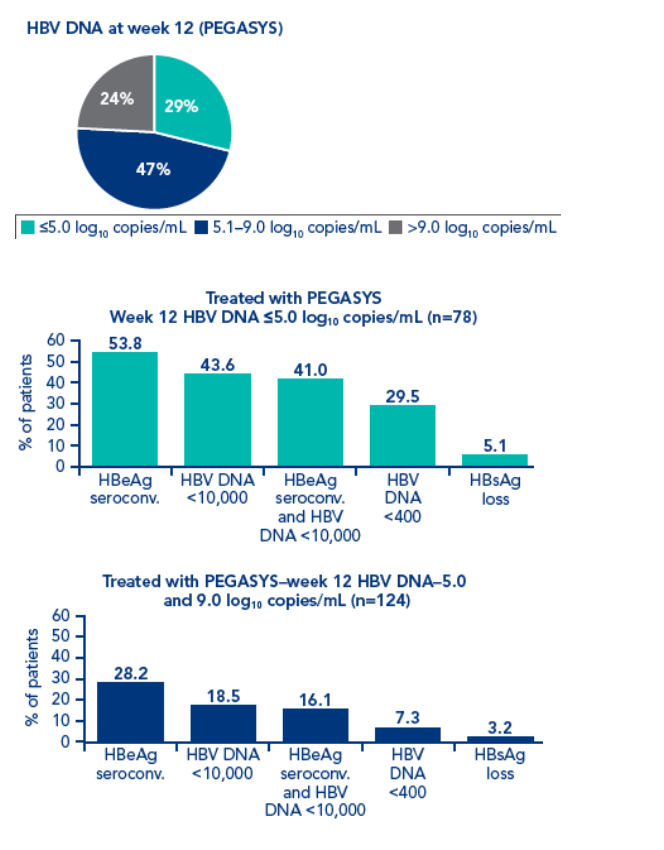
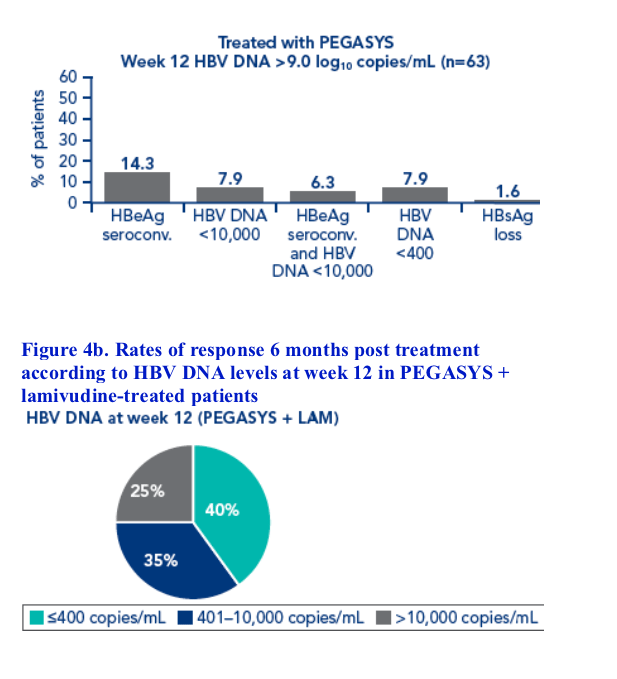
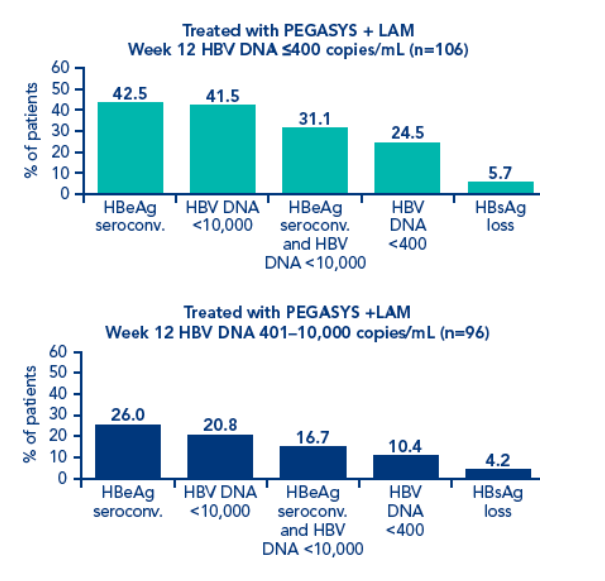
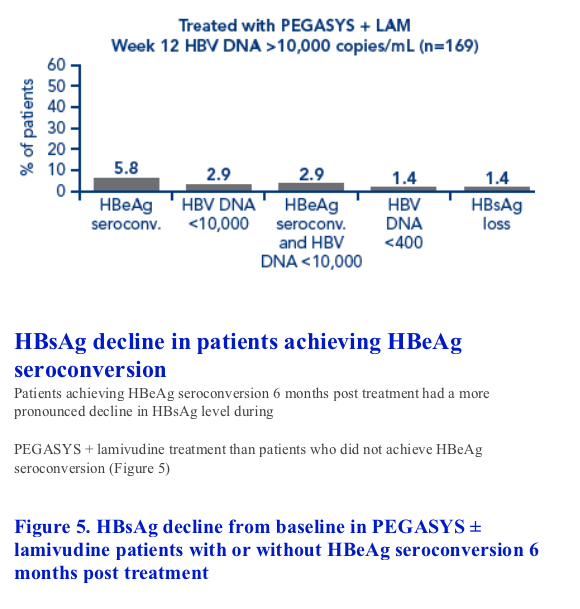
Patients with HBsAg values available at all timepoints
Of the patients with HBsAg ≦1500 at week 12 and HBeAg seroconversion 6 months post treatment, 10/55 (18%) and 7/47 (14.9%) also achieved HBsAg clearance 6 months and 1-year post treatment, respectively. Of the patients with HBsAg ≦1500 at week 24 and HBeAg seroconversion 6 months post treatment, 17/82 (20.7%) and 12/66 (18.2%) also achieved HBs Ag clearance 6 months and 1-year post treatment, respectively
In PEGASYS + lamivudine-treated patients with HBV DNA <400 copies/mL at week 12 and HBeAg seroconversion 6 months post treatment, 5/45 (11.1%) achieved HBsAg clearance 6 months post treatment. Patients with HBV DNA <5 log10 copies/mL at week 12 and HBeAg seroconversion 6 months post treatment, 4/42 (9%) achieved HBsAg clearance 6 months post treatment
References
[1. Korevaar A et al. High rates of HBsAg seroconversion in chronic hepatitis B patients responding to interferon therapy - a long-term follow-up study. AASLD 2007; P991
2. Lau GKK et al. Peginterferon alfa-2a, lamivudine and the combination for HBeAg-positive chronic hepatitis B. New Engl J Med 2005; 2682-2695
3. Janssen HL et al. Measurement of HBsAg to monitor hepatitis B viral replication in patients on alpha-interferon therapy. Antiviral Res 1994; 23: 251-257
4. Marcellin P et al. In patients with HBeAg-negative chronic hepatitis B, HBsAg serum levels early during treatment with peginterferon alfa-2a predict HBsAg clearance 4 years post treatment. Hepatology 2008; 48 (suppl 1): 919A
5. Lau GKK et al. Durability of response and occurrence of late response to PEGASYS] one year post-treatment in patients with HBeAg-positive chronic
hepatitis B. EASL; April 26-30, 2006; Vienna, Austria
Disclosure information: Editorial support for the development of this poster was funded by F. Hoffmann-La Roche, Basel, Switzerland
|
| |
|
 |
 |
|
|