 |
 |
 |
| |
Raltegravir Demonstrates Durable Efficacy Through 96 Weeks: Results from STARTMRK, A Phase III Study of Raltegravir-based vs. Efavirenz-based Therapy in Treatment-Naïve HIV+ Patients
|
| |
| |
Reported by Jules Levin
ICCAC Sept 12 2009 San Francisco
J. Lennox1, E. DeJesus2, A. Lazzarin3, D. Berger4, R. Pollard5, J. Madruga6, J. Zhao7, C. Gilbert7, A. Rodgers7, H. Teppler7, B-Y. Nguyen7, R. Leavitt7, and P. Sklar7 for the STARTMRK (P021) Investigators
1Emory University, Atlanta, GA, USA; 2Orlando Immunology Center, Orlando, FL, USA; 3University Vita-Salute San Raffaele, Milan, Italy; 4Northstar Medical Center, University of Illinois at Chicago, Chicago, IL, USA;
5University of California @ Davis, Sacramento, CA, USA; 6Centro de Referencia e Treinamento DST/AIDS, Sao Paulo, Brazil; 7Merck Research Labs, North Wales, PA, USA
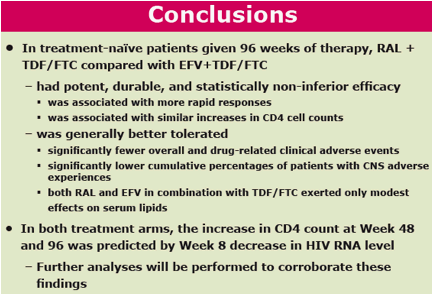
In treatment-naïve patients given 96 weeks of therapy, RAL + TDF/FTC compared with EFV+TDF/FTC
- had potent, durable, and statistically non-inferior efficacy
· was associated with more rapid responses
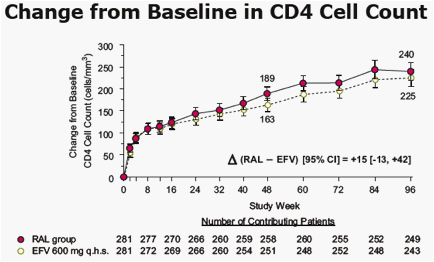
· was associated with similar increases in CD4 cell counts
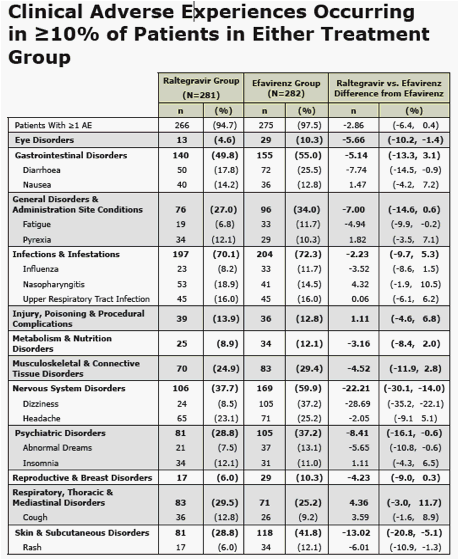
- was generally better tolerated
· significantly fewer overall and drug-related clinical adverse events
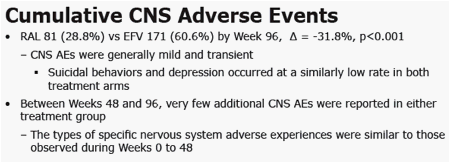
· significantly lower cumulative percentages of patients with CNS adverse experiences
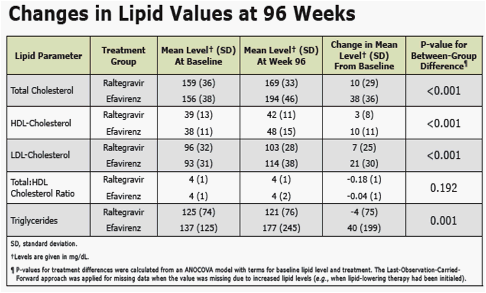
· both RAL and EFV in combination with TDF/FTC exerted only modest effects on serum lipids
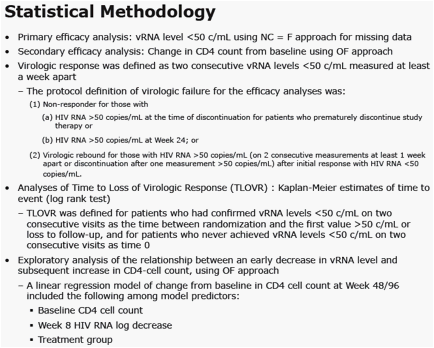
· In both treatment arms, the increase in CD4 count at Week 48 and 96 was predicted by Week 8 decrease in HIV RNA level
- Further analyses will be performed to corroborate these findings
ABSTRACT
Background: In STARTMRK, an ongoing, double-blind study, raltegravir (RAL) had potent and non-inferior antiretroviral activity compared to efavirenz (EFV) & was generally well tolerated through 48 weeks; RAL also showed more rapid time to HIV(v)RNA<50 c/mL than EFV.
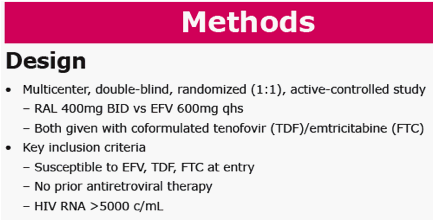
Methods: Patients with vRNA >5000 c/mL & no resistance to EFV, tenofovir (TDF) or emtricitabine (FTC) were randomized (1:1) to RAL (400 mg bid) or EFV (600 mg qhs), with TDF/FTC. Standard 96-week endpoints were evaluated. Exploratory analyses investigated the potential relationship between early virologic response and long-term CD4 response.
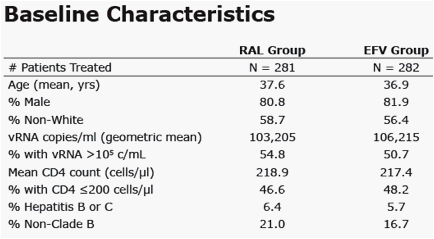
Results: Baseline characteristics were comparable. Results at Week 48 and 96 are shown:
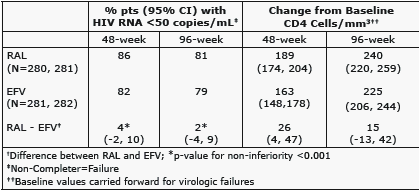
Conclusion: In treatment-naïve patients, RAL+TDF/FTC had durable, non-inferior antiretroviral activity sustained to 96-weeks compared to EFV+TDF/FTC & continued to be generally well tolerated.
BACKGROUND
Efficacy and Safety Results through Week 48
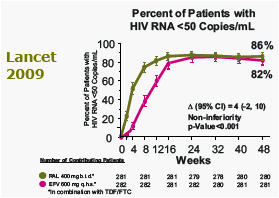
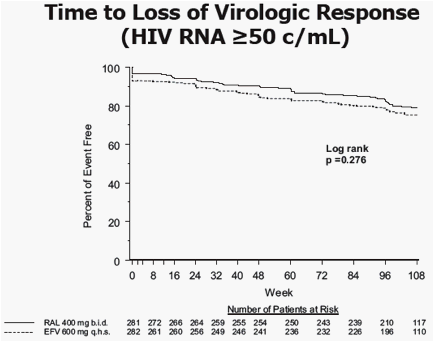
RAL provided potent and statistically non-inferior viral suppression compared to EFV
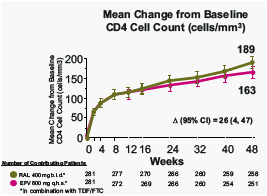
RAL exerted a greater immunological effect than EFV, measured by the increase in CD4 cell counts
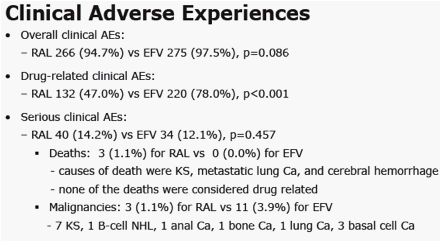
RAL was generally better tolerated than EFV
- significantly fewer overall and drug-related clinical adverse events
- significantly lower percentages of patients with CNS side-effects
Safety profile was similar in subjects with or without hepatitis B and/or hepatitis C
RAL had modest effects on serum lipids

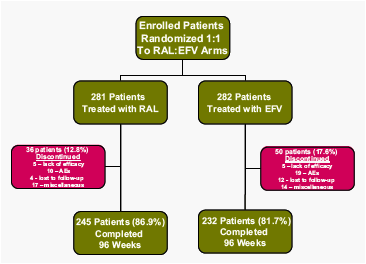
Patient Disposition at Week 96
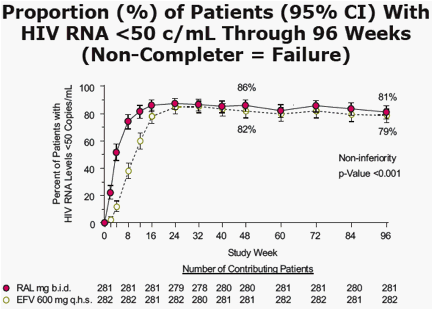
Proportion (%) of Patients With HIV RNA <400 c/mL At 96 Weeks (Non-Completer = Failure)
- RAL group 85% vs. EFV group 81%
- Non-inferiority p<0.001
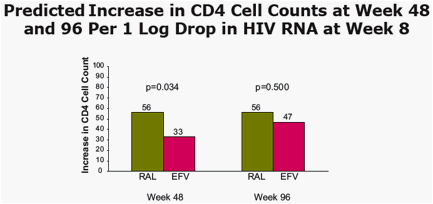
At week 48, statistically significant predictors of increase in CD4 count were baseline CD4 count, log drop in week 8 vRNA level, and treatment group.
At Week 96, statistically significant predictors of increase in CD4 count were baseline CD4 count and log drop in week 8 vRNA level.
|
| |
|
 |
 |
|
|