| |
HBV Viral Load/Fibrosis/ALT/Inflammation HBeAg+/HBeAg-: "Increasing hepatitis B viral load is associated with risk of significant liver fibrosis in HBeAg-negative but not HBeAg-positive chronic hepatitis B"
|
| |
| |
Download the PDF here
"Serum ALT was shown to be a useful marker of risk for both significant inflammation and fibrosis. Our findings of relatively low median ALTs (below 3 x ULN) in all HBeAg/DNA groups support the increasing recognition that even modestly elevated ALT levels can be associated with the risk of liver damage.....The odds of F2/3/4 fibrosis increased by 2.80 (95% CI 1.6-4.9) for every 10-fold increase in ALT (P<0.001). The odds of A2/3 inflammation increased by 2.90 (95% CI 1.51-5.58) for every 10-fold increase in ALT (P<0.001)."
Table 1. Demographical, biochemical, serological, virological and histological features of patients with chronic hepatitis B in HBeAg and DNA-based groups (n=394)
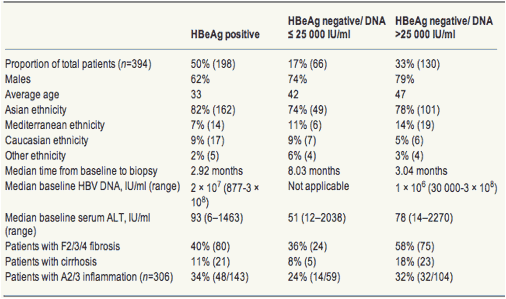
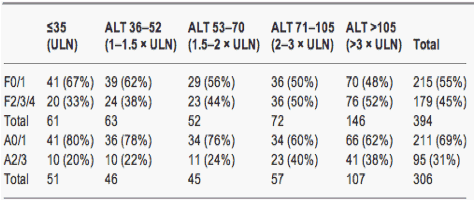
Table 4. Numbers (%) of patients with significant fibrosis and inflammation in strata of alanine aminotransferase from ≤upper limit of normal to >3 x upper limit of normal
Increasing hepatitis B viral load is associated with risk of significant liver fibrosis in HBeAg-negative but not HBeAg-positive chronic hepatitis B
Liver International May 2010
Catherine M. N. Croagh 1 , Sally J. Bell 1 , John Slavin 2 , Yu X. G. Kong 1 , Robert Y Chen 1 , Stephen Locarnini 3 and Paul V Desmond 1
1 Department of Gastroenterology, St Vincent's Hospital, Fitzroy, Vic., Australia
2 Department of Pathology, St Vincent's Hospital, Fitzroy, Vic., Australia
3 Victorian Infectious Diseases Reference Laboratory, North Melbourne, Vic., Australia
Correspondence
Catherine M. N. Croagh, MBBS, FRACP, MPH, Department of Gastroenterology, St Vincent's Hospital, Level 4, Daly Wing, 41, Victoria Parade, Fitzroy 3065, Vic., Australia
Tel: +61 03 9288 3580
Fax: +61 03 9288 3590
e-mail: catherine.croagh@svhm.org.au
ABSTRACT
Background/aims: To evaluate the association between demographical features, serum ALT and HBV DNA and the prevalence of significant fibrosis and inflammation on liver biopsy in patients with chronic hepatitis B.
Methods: In this cross-sectional study of patients on St Vincent's Hospital HBV database, patients were classified into three groups on the basis of HBeAg status and HBV DNA level and the prevalence of significant (F2/3/4) fibrosis and (A2/3) inflammation in each group was established. Patients were also divided into HBeAg-positive and -negative groups and examined for the prevalence of significant fibrosis/inflammation in the strata of HBV DNA and ALT. Predictors of significant fibrosis and inflammation in HBeAg-positive and -negative patients were examined by logistic regression.
Results: Three hundred and ninety four patients (HBeAg positive=198; HBeAg negative=196) with liver biopsy were identified. Fifty-eight percent of HBeAg-negative patients with HBV DNA >25 000 IU/ml had F2/3/4 fibrosis. HBV DNA and F2/3/4 were positively correlated in HBeAg-negative patients [odds ratio (OR) 1.42, P=0.001] but inversely correlated in HBeAg-positive patients (OR 0.71, P=0.03). HBV DNA was an independent predictor of significant fibrosis in HBeAg negative (P=0.03) but not HBeAg-positive patients. In HBeAg-positive patients, age was the only predictor of significant fibrosis (P=0.001) and ALT the only predictor of significant inflammation (P=0.003). In the whole cohort there was a close positive association between inflammation and fibrosis.
Conclusion: Increasing levels of HBV DNA are associated with increasing prevalence of significant fibrosis only in patients with HBeAg-negative CHB.
The development of advanced fibrosis or cirrhosis of the liver is one of the most significant sequelae of chronic hepatitis B (CHB) (1) The annual incidence of cirrhosis in patients with CHB has been estimated at 1.3-2.4% (2). Although the cumulative 5-year survival rate for patients with compensated cirrhosis is 84% (3), in patients with decompensated cirrhosis, this declines to 14-35% (3, 4). The prevalence of significant histological lesions has been shown to be high in biopsied cohorts (5) and active inflammation is thought to be the driving force to fibrosis (6).
A number of studies have examined predictors of progression to advanced fibrosis (7-11) and considerable attention has been focused on a large, prospective study of the incidence of cirrhosis in CHB published by the REVEAL group (12). This study followed 3582 subjects for a mean of 11 years and reported that cirrhosis was strongly correlated with the level of circulating virus, with an increasing incidence of cirrhosis being found in patients with higher levels of virus at entry into this study. It should be noted that 85% of this cohort was HBeAg negative and this study was restricted to patients over 30 years of age. Cirrhosis was diagnosed on the basis of ultrasound. It is unclear whether these data can be applied to younger, HBeAg-positive patients.
The current literature in HBeAg-positive patients does not support an association between HBV DNA and fibrosis. Two studies of over 300 predominantly HBeAg-positive patients did not find HBV DNA to be a predictor of fibrosis (13, 14) In fact, in HBeAg-positive patients, it has been suggested that lower HBV DNA, reflecting an increased immune response, is correlated with fibrosis. Wang et al. (15) studied 28 HBeAg-positive, immune tolerant patients and reported that lower serum HBV DNA level, along with age >30 years, was independently correlated with stage 2 fibrosis or more on liver biopsy.
In recent clinical practice there has been a focus on HBV DNA levels as the trigger for antiviral therapy. We postulated that this is only appropriate in patients with HBeAg-negative CHB.
Aims
The aims of our study were:
* 1. to evaluate the prevalence of significant fibrosis, cirrhosis and inflammation in a well-characterised cohort of HBeAg-positive and HBeAg-negative patients who had undergone liver biopsy, divided into three groups according to the phase of the disease.
* 2. to examine the relationship between inflammation and fibrosis on an individual level.
* 3. to evaluate the interaction between HBeAg status, HBV DNA, ALT and significant histological fibrosis and inflammation.
* 4. to identify predictors of significant fibrosis, inflammation and cirrhosis in HBeAg-positive and -negative groups.
Discussion
This study demonstrates that progressive liver disease in CHB is driven by three main factors: age, a surrogate of disease duration; immune activation, as measured by ALT; and viral replication, measured by HBV DNA levels. However, the influence of HBV DNA levels differs in HBeAg-positive and -negative disease. Increasing HBV DNA decreased the odds of significant fibrosis in the HBeAg-positive patients and increased the odds of significant fibrosis in the HBeAg-negative group. This highlights the fact that the significance of HBV DNA in terms of fibrosis risk is best considered in the context of the patient's phase of disease and HBeAg status.
The natural history of perinatally acquired CHB may be classified into four phases based on HBeAg status, HBV DNA, ALT level and histology: the HBeAg-positive immune tolerant and immune clearance phases and the HBeAg-negative immune control/residual inactive phase and immune escape/reactive immune clearance phases (17, 18). HBeAg-positive patients in the immune tolerant phase usually have very high HBV DNAs and little hepatic inflammation or fibrosis (19) as was seen in this cohort (see the schema for explaining the relationship of HBV DNA and fibrosis, Fig. 2). In the immune clearance phase, the host clears the virus and removes hepatocytes, resulting in the development of fibrosis (20). Accordingly, the HBeAg-positive patients with the lowest HBV DNA (<100 000 IU/ml) had the highest prevalence overall of F2/3/4 and A2/3 at 75 and 71%, although the numbers in this group were small (Fig. 2). In the HBeAg-negative patients those with a low HBV DNA (ENLR) have a low prevalence of F2/3/4 and may be considered to be in a state of immune control. Liver injury is not marked in these ENLR patients as they may have transitioned to this phase after a short immune clearance phase and also because of the regression of fibrosis over time in this phase (21). HBeAg-negative patients with higher HBV DNA levels (ENHR) may be considered to be in the immune escape phase of disease and it is proposed that the higher prevalence of F2/3/4 in this group is the cumulative effect of injury sustained during the immune clearance phase and the ongoing inflammation in HBeAg-negative CHB (Fig. 2).
These data confirm the results of the REVEAL study, which showed a clear relationship between increasing HBV DNA and fibrosis in a mainly HBeAg-negative CHB cohort over 30. However, our study differs from REVEAL in that all patients had histological quantification of fibrosis rather than ultrasound. In addition, over half the patients had HBeAg-positive disease, and in these patients, high HBV DNA was not associated with fibrosis.
The strong predictive power of age as a marker of significant fibrosis in HBeAg-positive disease leads to proposing that older age may signify a protracted immune clearance phase with repeated unsuccessful attempts at viral clearance. This cannot, however, be known with certainty from cross-sectional data. A longer immune tolerant phase with a severe flare on entry into immune clearance could result in a similar outcome.
Serum ALT was shown to be a useful marker of risk for both significant inflammation and fibrosis. Our findings of relatively low median ALTs (below 3 x ULN) in all HBeAg/DNA groups support the increasing recognition that even modestly elevated ALT levels can be associated with the risk of liver damage (22).
In this study, only three of the four phases of disease were truly represented. All HBeAg-positive patients were considered together because numbers in the immune tolerant group would have been quite small primarily because liver biopsies are not often performed in this group. An HBV DNA threshold of 25 000 IU/ml was used to distinguish between HBeAg-negative phases of disease because this was the lower limit of detection of the relatively insensitive Digene II assay on which over half the cohort had been tested and we acknowledge that this is a higher cut-off point than recent recommendations (23).
Although this is a cross-sectional study, significant differences in the ages between patient groups were observed, suggesting a transition from HBeAg-positive disease to a HBeAg-negative non or low replicative state (ENLR) over years, followed by relapse to HBeAg-negative chronic hepatitis (ENHR) as has been observed in longitudinal studies (24). The ENHR group has a preponderance of males, and the high proportion of Asians in this group suggests that HBeAg-negative CHB is common in Asian populations as has been increasingly recognised (25).
Our cohort is a heterogeneous one, with all major genotypes (A-D) being represented.
The high prevalence of F2/3/4 fibrosis in ENHR patients is in keeping with other recent studies that have reported a high prevalence of histological indications for treatment in HBeAg-negative patients (5); however, it must be acknowledged that the biopsied group presented here is biased towards patients with a higher likelihood of advanced fibrosis because clinicians used markers of higher ALT and signs of chronic liver disease on examination to guide the decision to biopsy. The finding of moderate and severe inflammation in the majority of patients with F3 or F4 fibrosis highlights that viral suppression with resultant reduction in inflammation is very important in patients with advanced fibrosis (26).
Conclusions
Our study of an Australian clinical cohort confirms that CHB is associated with relatively high rates of significant fibrosis, especially among ENHR patients, representing the immune escape phase of disease or chronic HBeAg-negative Hepatitis B. We confirm that there is a positive correlation between high HBV DNA levels and the presence of significant liver fibrosis in HBeAg-negative disease but not in HBeAg-positive disease, and outline a potential explanation for this based on the underlying phase of disease. The factors predictive of significant histological disease were age in HBeAg-positive patients, and both HBV DNA and ALT in HBeAg-negative patients.
The risk of liver inflammation and fibrosis in chronic hepatitis B is the complex result of many interacting factors and we argue that it is best considered in the context of the traditional phase of disease model rather than as a simple function of any single factor alone. In particular, the HBV DNA level should not be relied on in isolation to predict significant fibrosis/inflammation, but rather needs to be interpreted as a measure of risk in the context of other factors especially HBeAg status.
Results
Patient characteristics
Three hundred and ninety-four (HBeAg positive, n=198 and HBeAg negative, n=196) patients were available for analysis of histological fibrosis and 306 (HBeAg positive, n=143 and HBeAg negative, n=163) for inflammation. One hundred and fifty-five patients were tested with the Bayer Versant 3.0 HBV DNA assay and 239 patients with the Digene hybridisation capture assay. The distribution between the patients in the three HBeAg and DNA-based groups showed that 50% of patients were EP, 17% were ENLR and 33% were ENHR (Table 1). The ethnic makeup of these groups did not differ significantly (P=0.189), with the majority (>74%) of patients being of Asian ethnicity in all the groups. The gender proportions of the three groups, varied with significantly more males in the ENHR (79%) than that in the EP group (62%) (P=0.001).
HBeAg-positive patients were the youngest (mean 33 years) and the ENHR were the oldest (mean 47 years), with ENLR patients being intermediate (mean 42 years) (P<0.001). Using post hoc pairwise comparisons, the differences of means between the EP and ENLR (9.1 years), EP and ENHR (13.6 years) as well as ENLR and ENHR (4.6 years) groups were all highly statistically significant (P<0.001).
The serum HBV DNA was significantly higher in EP patients than that in ENHR patients (P<0.001), and the ALT was also significantly different in the three groups (P<0.001), with the EP patients having the highest ALT and the ENLR having the lowest ALT levels (Table 1).
Patient characteristics - genotype
Two hundred and two patients had genotyping available. 110 (54%) were in the EP group, 72 (36%) in the ENHR and 20 (10%) in the ENLR group (Table 2). The most common genotypes were B and C, accounting for 37% of the total group each. Genotype D represented 16% of the group and genotype A 6%. A small number of patients had mixed genotypes: B/C in six cases and C/D in one case. The predominant genotype among EP patients was genotype C, representing 45% of the group, followed by genotype B with 33%. Genotypes D and A represented 12 and 8%, respectively, in this group.
Seventy percent of the ENLR group was genotype B and 20% genotype D. There was only one genotype C patient in the ENLR group and the only genotype G patient was also in the ENLR group. Genotypes B and C were the most common in the ENHR group at 35 and 33% respectively.
Patients with genotypes B and C were of Asian ethnicity in over 95% of the cases. Genotype A patients were predominantly Caucasian (58%) and 33% were Asian, with the remaining 8% being of Mediterranean origin. Genotype D patients were predominantly Mediterranean (73% of cases), with minorities also being Asian and Caucasian.
Prevalence of significant fibrosis and inflammation in HBeAg/HBV DNA groups
The highest prevalence of F2/3/4 fibrosis was found in the ENHR group at 58% (Table 1). This was significantly higher than that in the EP group of 40% (P=0.002) and in the ENLR group of 36% (P=0.005). The prevalences of F2/3/4 fibrosis in the EP and ENLR groups were not significantly different (P=0.561). The prevalences of cirrhosis in the EP, ENLR and ENHR groups were 11, 8 and 18%, respectively, but these differences did not reach statistical significance (P=0.069). The prevalence of A2/3 inflammation was also not significantly different among the three groups, being 34, 24 and 32% in the EP, ENLR and ENHR groups respectively (P=0.382).
Relationship between histological inflammation and fibrosis scores
Increasing grades of inflammation were strongly associated with increasing degrees of fibrosis on liver biopsies (P<0.001) (Fig. 1). No patients with F0 fibrosis had moderate or severe inflammation, while 76% of cirrhotic patients had A2 or A3 inflammation.
Fig. 1. Correlation between inflammation and fibrosis scores on liver biopsies in chronic hepatitis B patients (n=306).
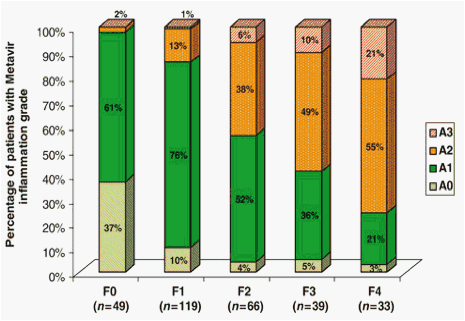
The positive correlation between inflammation and fibrosis was also seen in analyses confined to both the HBeAg-positive (n=143) (P<0.001) and the HBeAg-negative groups (n=163) (P<0.001). The mean Metavir activity score also increased with increasing fibrosis, being 0.65, 1.05, 1.45, 1.64 and 1.95 in patients with F0, F1, F2, F3 and F4 fibrosis respectively.
Prevalence of significant fibrosis and inflammation in different strata of HBV DNA in HBeAg-positive and -negative disease
The prevalence of F2/3/4 fibrosis increased from 38% in HBeAg-negative patients with HBV DNA of <105IU/ml to 71% in those with HBV DNA of ≥107IU/ml (Table 3). In HBeAg-positive patients, however, the pattern was the reverse; 75% of patients in the HBV DNA strata of <105 IU/ml had F2/3/4 fibrosis while only 38% in the strata of HBV DNA ≥107 IU/ml did.
Table 3. Numbers (%) of patients with significant fibrosis and inflammation in strata of hepatitis B virus DNA from <105 to ≥107 according to HBeAg status
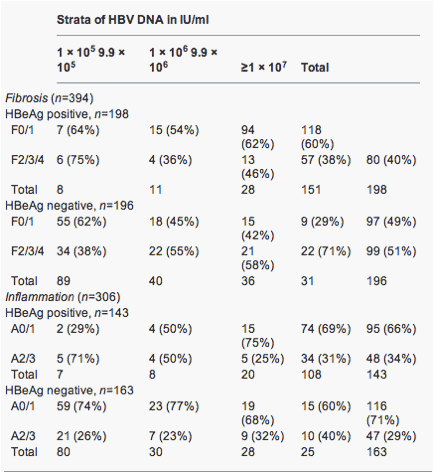
Using logistic regression, a significant interaction was found between the HBeAg status and HBV DNA level, with F2/3/4 fibrosis as the outcome (P<0.001). In HBeAg-negative patients, the odds of F2/3/4 increased by 1.42 [95% confidence interval (CI) 1.16-1.74] for every 1 log increase in HBV DNA (P=0.001). In contrast, in HBeAg-positive patients, the odds of F2/3/4 decreased: odds ratio (OR) 0.71 (0.52-0.97) per 1 log increase in HBV DNA (P=0.03). Thus, the association between HBV DNA and F2/3/4 was positive in HBeAg-negative disease, but negative in HBeAg-positive disease.
The prevalence of A2/3 inflammation also increased from 26% in HBeAg-negative patients with HBV DNA of <105 IU/ml to 40% in those with HBV DNA of ≥107 IU/ml (Table 3). In HBeAg-positive patients, however, the pattern was the reverse; 71% of patients in the HBV DNA strata of <105 IU/ml had A2/3 while only 31% in the strata of HBV DNA≥107 IU/ml did.
Using logistic regression, a significant interaction was found between the HBeAg status and HBV DNA, with A2/3 inflammation as the outcome (P=0.013). In HBeAg-negative patients, the OR of A2/3 increased by 1.17 (0.93-1.48) for every 1 log increase in HBV DNA (P=0.18). In HBeAg-positive patients, the odds of A2/3 decreased 0.69 (0.49-0.98) for every 1 log increase in HBV DNA (P=0.035)
Prevalence of significant fibrosis and inflammation in different strata of alanine aminotransferase in HBeAg-positive and -negative disease
The interaction between ALT and the presence of either F2/3/4 or A2/3 was not significantly different in HBeAg-positive and -negative groups (P=0.096 and 0.339 respectively). Therefore the prevalence of F2/3/4 and A2/3 according to the strata of ALT was examined in the overall cohort. The prevalence of F2/3/4 was the lowest in patients with an ALT below ULN at 33% and the highest in patients with ALT >3 x ULN at 52% (Table 4). A2/3 inflammation in the overall cohort was the lowest in patients with an ALT below ULN (20%) and the highest in patients with ALT >2 x ULN (38-40%) (Table 4).
Table 4. Numbers (%) of patients with significant fibrosis and inflammation in strata of alanine aminotransferase from ≤upper limit of normal to >3 x upper limit of normal
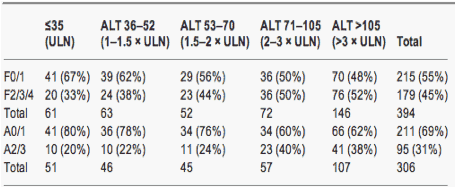
The odds of F2/3/4 fibrosis increased by 2.80 (95% CI 1.6-4.9) for every 10-fold increase in ALT (P<0.001). The odds of A2/3 inflammation increased by 2.90 (95% CI 1.51-5.58) for every 10-fold increase in ALT (P<0.001).
Predictors of significant fibrosis and inflammation in patients in HBeAg-positive and -negative disease
Using logistic regression (see 'Methods') in the HBeAg-positive group, the only predictor of significant fibrosis was age (P<0.001). The odds of F2/3/4 increase by 1.057 for each increase in age of one year or 1.73 for an increase of 10 years. The proportion of HBeAg-positive patients with F2/3/4 in groups aged <20, 20-29, 30-39, 40-49, 50-59 and≥60 years was 10, 30, 41, 56, 63 and 80% respectively. The number of patients in these groups was 10, 79, 56, 32, 16 and 5 respectively.
In the HBeAg-negative group, the predictors of significant fibrosis were HBV DNA, which had an OR of 1.3 for every 1 log increase in HBV DNA (P=0.03) and ALT, with an OR of 2.9 for every 10-fold increase in ALT (P=0.02).
In the HBeAg-positive group, ALT was an independent predictor of A2/3 (P=0.003), with an Odds Ratio of 5.33 for every 10-fold increase in ALT. In HBeAg-negative disease, there were no statistically significant predictors of A2/3 at the 5% level.
Predictors of cirrhosis in patients with HBeAg-positive and -negative chronic hepatitis B
A further logistic regression (see 'Methods') was fitted for HBeAg-positive (n=21) and -negative (n=28) patients with cirrhosis (F4 fibrosis) as the outcome.
In the HBeAg-positive group, once again, age was a strong predictor of cirrhosis (P=0.001). Lower HBV DNA was also found to be a predictor, with a significant negative association being seen between HBV DNA and cirrhosis (P=0.002). The OR for every 1 log increase in HBV DNA was 0.498. In the HBeAg-negative group, the only significant variable was age (P=0.03).
Methods
Patients
St Vincent's Hospital, Melbourne, has maintained a database of CHB patients seen in their liver clinics since 1996. CHB patients on whom a full set of baseline demographic (including date of birth, sex, ethnic origin and country of birth), serological, virological and histological details was available as of 1 October 2008 were included for analysis. The baseline clinic recordings of HBV serology including HBeAg and antibody (anti-HBe) as well as HBV DNA levels and serum ALT levels were used. Liver biopsy was performed pre-treatment at the discretion of the clinician and in some situations had been performed before the patient was seen at the liver clinic. In Australia, liver biopsy is a requirement for subsidised antiviral therapy, and therefore most liver biopsies were performed in patients being assessed for treatment. For analyses of patient characteristics and the overall prevalence of significant fibrosis/inflammation and cirrhosis, the patients were divided into three groups on the basis of HBeAg status and HBV DNA level:
*
HBeAg positive/DNA detected [HBeAg positive (EP); immune tolerant and immune clearance phase].
*
HBeAg negative/DNA ≤25 000 IU/ml [HBeAg-negative low replicators (ENLR) or immune control phase].
*
HBeAg negative/DNA >25 000 IU/ml [HBeAg-negative high replicators (ENHR); immune escape phase].
For other analyses, the total cohort was simply divided according to HBeAg status. The prevalence of significant fibrosis and inflammation was examined separately in HBeAg-positive and -negative groups in strata of HBV DNA increasing in 1 log increments from <105 to ≥107 IU/ml and in strata of ALT increasing from less than or equal to the upper limit of normal (ULN) to >3 x ULN. The ULN for ALT was defined as 35 IU/ml. This study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the St Vincent's Hospital Human Research and Ethics Committee.
Hepatitis B virus serological and DNA testing
Hepatitis B surface antigen was measured using a commercially available immunoassay (Abbott Laboratories, North Chicago, IL, USA) and HBeAg and anti-HBe by an immunoassay produced by BioMerieux Clinical Diagnostics (Marcy l'Etoile, France).
All tests pertaining to HBV DNA were performed at the Victorian Infectious Diseases Reference Laboratory. Before April 2004, HBV DNA levels were performed using a capture hybridisation assay (Digene Hybrid Capture, Digene Diagnostics Inc., Beltsville, MD, USA) with a lower limit of detection of 0.5 pg/ml (approximately 25 000 IU/ml). Patients tested using the Digene assay who were below the limit of detection were assigned the value of 25 000 IU/ml. Any patient with an undetectable HBV DNA on Hybridisation assay before January 2000 was excluded unless a concomitant PCR was also negative to ensure the exclusion of older generation, less sensitive hybridisation assays. Subsequent to April 2004, the HBV DNA viral load was assessed by a bDNA signal amplification probe method (Bayer Versant HBV DNA 3.0 assay). The dynamic range is 351-17 857 140 IU/ml. 1 IU/ml is equivalent to 5.6 copies/ml. Patients tested using the bDNA assay whose HBV DNA levels were below and above the limits of detection were assigned the values of 351 and 17 857 140 IU/ml, respectively, for analyses involving HBV DNA.
Liver biopsy
Liver biopsies are performed with a 15G Biopty™ gun (Bard, Covington, GA, USA) under ultrasound guidance with usually one pass, the average length being 18 mm. The usual number of portal tracts considered adequate by the pathology department on which to report a biopsy is 11-15. Formalin-fixed, paraffin-embedded liver biopsies were sectioned and stained according to usual histology methods. The routine stains performed included haematoxylin and eosin for general liver histology and a reticulin and Picro-Mallory trichrome stain for demonstrating fibrous connective tissue. The biopsies were evaluated by hospital pathologists.
The histological lesions were graded according to the classification proposed by the Metavir study group (16). Activity is graded applying an algorithm that includes the severity of piecemeal and lobular necrosis, resulting in a scale of 0-3 (0=none, 1=mild, 2=moderate and 3=severe activity). Significant inflammation was defined as activity scores of A2/3. Activity scores were not available on the database on 88 patients; thus, the number available for analysis of inflammation was 306.
Fibrosis is scored from 0 to 4 (0=no fibrosis, 1=enlarged fibrotic portal tracts, 2=periportal or portal-portal septa but intact architecture, 3=fibrosis with architectural distortion but no obvious cirrhosis, 4=probable or definite cirrhosis). Significant fibrosis was defined as a fibrosis score of F≥2. Biopsies in which fibrosis had initially been staged using an alternative staging method (e.g. Scheuer's) were revised into the metavir scoring system.
Statistical analysis
Data entry and statistical analysis were performed with the statistical package spss (version 17.0, SPSS Inc., Chicago, IL, USA). The corrected χ2-test or two-sided Fisher's exact test was used to compare categorical data, while Student's t-test or one-way anova was used for group comparisons of parametric quantitative data and the Mann-Whitney or Kruskal-Wallis test for similar comparisons of non-parametric data. Logistic regression models were used for multivariable analysis. Logistic regression models were fitted separately for HBeAg-positive and -negative patients with significant fibrosis, inflammation and cirrhosis as the outcomes and the following as potential explanatory variables: age, gender, ethnicity, HBV DNA and ALT. Results were presented as mean or median whenever appropriate. In all cases, tests of significance were two-tailed with a level at <0.05.
|
|
| |
| |
|
|
|