 |
 |
 |
| |
Efficacy and Safety of Entecavir Treatment in a Heterogeneous CHB Population in a Real-Life Setting in China
|
| |
| |
Reported by Jules Levin
21st APASL, 17-20 February 2011, Bangkok
J. Hou1, H. Ren2, Y. Wang3, Q. Xie4, D. Tan5, W. Zhao6, C. Llamoso7, D. Cohen7, M. Wu8
1Nanfang Hospital, Southern Medical University, Guangzhou, China; 2Second Affiliated Hospital of Chongqing Medical University, Chongqing Medical University, China; 3First Affiliated Hospital of the Third Military Medical University, Chongqing, China;4Ruijin Hospital, Shanghai Jiaotong University, Shanghai, China; 5Second Xiang Ya Hospital, Central South University, Changsha, China; 6Nanjing No.2 Hospital, Nanjing, China, 7Research & Development, Bristol-Myers Squibb, Wallingford, MA, USA,8Research & Development, Bristol-Myers Squibb, Shanghai, China
Author Conclusions
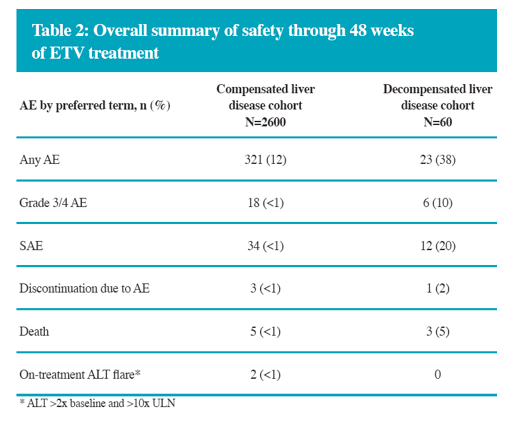
In the real-life setting ETV was efficacious and well tolerated through 48 weeks of treatment in a heterogeneous population of Chinese patients with CHB
The overall safety profile of ETV for both compensated and decompensated liver disease in this substudy is comparable to the safety profile established in China and global registrational studies
The efficacy data are consistent with results from China and global registrational studies
Introduction
· Entecavir (ETV) has demonstrated superior histologic, virologic, and biochemical activity compared with lamivudine (LVD) at Week 48 innucleoside-naive and LVD-refractory patients with chronic hepatitis B (CHB) in global registrational studies (ETV-022, ETV-026, ETV-027)1-3
· In China, ETV also demonstrated superior efficacy to LVD for virologic and biochemical endpoints at Week 48 in nucleoside-naive and LVD-refractory Chinese CHB patients (ETV-023 and ETV-056)4,5
· Clinical studies have shown ETV to be well tolerated and to have a favorable safety profile through up to 6 years of treatment 1-6
· We present here Week 48 results from the China substudy of the REALMstudy, assessing the efficacy and safety of ETV in a heterogeneous CHB population in a real-life clinical practice setting in China


References
1. Chang T-T, et al. N Engl J Med 2006; 354:1001-1010.
2. Sherman M, et al. Gastroenterology 2006; 130:2039-2049.
3. Lai C-L, et al. N Engl J Med 2006; 354:1011-1020.
4. Yao G, et al. Hepatol Int 2007; 1:365-372.
5. Yao G, et al. Hepatol Int 2007; 1:373-381.
6. Chang T-T, et al. Hepatology 2010; 52:886-893.
|
| |
|
 |
 |
|
|