| |
Natural history of chronic hepatitis B: what exactly has REVEAL Revealed? "Serum HBV DNA level was identified as the most important risk predictor for progressing to end-stage liver disease outcomes"
|
| |
| |
Download the PDF here
Liver International Early View
Article first published online: 17 APR 2012
"The Risk Evaluation of Viral Load Elevation and Associated Liver
Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) Study was initiated to address this very question with particular focus on the role of quantitative HBV DNA levels in predicting liver disease progression."
"Data from the REVEAL-HBV study have helped establish an HBV viral load paradigm in the natural history of chronic hepatitis B [8, 35, 36, 37]. Serum HBV DNA level was identified as the most important risk predictor for progressing to end-stage liver disease outcomes [17, 18, 38]. A fairly significant finding was the biological gradient of disease progression risk across the serum HBV DNA levels from a single measurement; in the case of progression to HCC, this risk gradient was found to be even more striking among chronic HBV carriers who were HBeAg-seronegative with normal ALT levels (see table 3 for HBV DNA levels & risk)......Our inference therefore is that the study entry single HBV DNA in this population accurately represented what must have been a prolonged period of HBV elevation; this may be particularly more relevant to the patients with childhood HBV infection. Importantly, these data cannot be viewed to directly reflect what will happen in other types of HBV infected populations."
"Some key takeaways from these analyses are as follows (i) a substantial drop in HBV DNA defined as ≥3 logs is associated with a 4-fold greater likelihood of HBsAg seroclearance compared with those who had a lower drop; (ii) Among subjects that cleared sAg, there was a lag of 4.6 year between the first measured significant drop in HBV DNA and HBsAg seroclearance; (iii) for the few subjects that came in with HBV DNA ≥ 10 000 copies/ml (2000 IU) and were able to totally suppress HBV DNA (<300 copies/ml) before HBsAg seroclearance or censoring, there was a very high rate of HBsAg seroclearance (25.8% at 60 months and 51.3% at 100 months; slope 0.5% per month). Another interesting observation was that having a mildly elevated ALT at the time of complete suppression of HBV DNA was associated with a greater likelihood of clearing the HBsAg; potentially an indication of an active immune system. These findings are consistent with previously published literature showing that within randomized clinical trials, subjects with post-treatment HBV-DNA level of <1000 copies/ml had a higher likelihood of achieving HBsAg loss"
"Those with the precore G1896 (wild-type) variant had a higher HCC incidence than for those with the G1896A variant (956 vs.269 per 100 000 person-years); and those with the BCP A1762T/G1764A double mutant had a higher HCC incidence than those with BCP A1762/G1764 (wild-type) variant (1149 vs. 359 per 100 000 person-years). The adjusted hazard ratio (95% CI) of developing HCC was 1.76 (1.19-2.61) for genotype C vs. genotype B; 0.34 (0.21-0.57) for precore G1896A vs. wild-type; and 1.73 (1.13-2.67) for BCP A1762T/G1764A vs. wild-type; serum HBV DNA level remained an independent predictor of HCC."
ABSTRACT
Chronic hepatitis B virus (HBV) infection is a serious public health problem because of its worldwide prevalence and potential to cause adverse consequences. The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study carried out in Taiwan was used to investigate the natural history of chronic hepatitis B. The REVEAL-HBV study has established an HBV viral load paradigm in the natural history of chronic hepatitis B (CHB). Serum HBV DNA level has been shown to be significantly and independently associated with incidence of hepatocellular carcinoma (HCC) and cirrhosis and liver-related mortality across a biological gradient. It is also a major predictor of HBsAg seroclearance.
- Genetic features including HBV genotype and basal core promoter A1762T/G1764A mutant, and precore G1896A mutant were documented as predictors of HCC risk.
- Inactive HBV carriers still had an increased risk on HCC development and liver-related mortality compared with HBsAg -seronegatives.
- Nomograms focusing on facilitating risk communication between patients and clinicians were developed incorporating non-invasive clinical parameters to predict long-term HCC risk. These will hopefully contribute to evidence-based decisions in the clinical management of CHB patients. A somewhat provocative and novel finding from the REVEAL-HBV study is the association of chronic HBV infection in active replication with an increased pancreatic cancer risk especially in women less than 50 years old. This finding will hopefully spur further research in this area seeking confirmatory evidence. Finally, we hope that the REVEAL-HBV study will continue to be a source of data to answer other important questions in chronic hepatitis B research going forward.
"However, the lack of association in some subgroups/categories may be because of small numbers of pancreatic cancer cases, thus the possibility of type II error should be taken into consideration when interpreting these data. Although no causal inference can be made, these results not only support an association between chronic HBV infection with active replication and increased pancreatic cancer risk, but represent the most provocative finding from the REVEAL-HBV study to date."
Background and overview
The hepatitis B virus (HBV) is a human carcinogen and chronic infection with the virus remains a major global health problem [1, 2]. Effective vaccines have been available since 1982 with strong evidence of public health benefits such as reduction in infection rates, mortality from infant fulminant hepatitis, chronic carrier rates and on the incidence of hepatocellular carcinoma (HCC) in children [3, 4, 5]. However, a third of the world's population (2 billion people) have been infected by this virus and more than 350 million people are estimated to be currently chronically infected [1, 2]. The overwhelming majority reside in the Asia-Pacific and sub-Saharan Africa regions [6, 7].
From previous studies the natural history of CHB involves three key phases; these phases were defined based upon a combination of serum markers such as serum alanine aminotransferase (ALT) level, hepatitis B e antigen (HBeAg), antibody against hepatitis B e antigen (anti-HBe), serum HBV load (mostly based upon branched-DNA assays) and hepatitis B surface antigen (HBsAg) [8, 9, 10, 11]. The three phases are: immune tolerance, immune clearance and the residual/inactive carrier phases. The immune tolerance phase can be very long lasting and is characterized by presence of HBeAg, and high level of HBV DNA in serum, normal-ALT levels and little to no evidence of liver injury. Most of the liver injury occurs during the immune detection and clearance phase as the host immune system tries to clear infected hepatocytes through specific T-lymphocyte-mediated cellular responses to viral antigens and apoptosis of hepatocytes. This results in hepatic inflammation, elevation of serum ALT levels, reduction of the circulating HBV DNA level, and hopefully seroconversion of HBeAg. The prognosis of long-term liver health is dependent among other things on the duration and intensity of the hepatocyte injury and regeneration cycle with resultant necroinflammation and fibrosis that occur during this phase. Finally, a proportion of infected persons are able to inactivate the replication of HBV and enter the residual (sometimes referred to as the "inactive carrier state") phase. This phase is characterized by the continued presence of HBsAg in serum, absence of HBeAg and the presence of anti-HBe antibody, low/undetectable levels of serum HBV DNA and normal ALT. Although rare, a very small proportion of infected persons are able to spontaneously clear the HBsAg and end up with resolution of the infection. Patients in the inactive carrier state usually have a low risk of liver disease progression, but this may depend on any pre-existing livery injury suffered in the immune detection/clearance phase.
Although the phases of the natural history of chronic HBV infection have been well characterized, the predictors of progression to liver injury and the relative importance of the different identified risk factors continue to be active areas of inquiry. The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) Study was initiated to address this very question with particular focus on the role of quantitative HBV DNA levels in predicting liver disease progression. This article reviews results from the REVEAL-HBV Study, building on a previously published review[9] and provides updated insights and perspectives based on the published peer-reviewed articles available at the time of preparation of this article. An overview of the key findings will be discussed below; however full presentations of the study results are available in the previously published primary papers.
Precursor to the REVEAL-HBV study: HBeAg serostatus and risk of HCC
The HBeAg is a marker of HBV replication; its seroprevalence in chronic HBV infected persons decreases with age [12]. Consistent with the natural history of chronic hepatitis B infection, the HBeAg seropositive rate is very high in early childhood infections; the seroprevalence rate drops from 64% in infected persons between 5-9 years old to 6% in patients 60-69 years old. The role of HBeAg as a risk factor for HCC development had been previously shown[12]; a meta-analysis of age-matched case-control studies showed a 3.7-fold greater risk of developing HCC with the presence of HBeAg in serum in chronic HBV infected persons [12]. In 2002, we published the results of a community-based cohort study of 11 893 men in Taiwan (Taiwan Community-Based Cancer Screening Project-pre-cursor to the REVEAL-HBV cohort study) [13], in which we observed a 6-fold increase in risk of HCC in men HBeAg+ compared with men HBeAg-. The significant finding on HBeAg seropositivity and increased HCC risk was further confirmed in analyses stratified by serum ALT level and cirrhosis status at study entry [12, 14].
In further analyses, a nested case-control analysis was also carried out to assess the impact of baseline HBV DNA measured by branched chain DNA assay and the risk of developing HCC in HBeAg-seronegative persons [13]. This analysis identified a significant dose-response relationship between serum HBV DNA level and risk of HCC (P = 0.005 for trend test).
Consequently, we postulated that a better measurement of the viral load such as a polymerase chain reaction assay, might provide better predictability of the role of HBV viral load in liver disease progression in general and HCC in particular. This led to the REVEAL-HBV study [8].
The REVEAL-HBV study
Full descriptions of the study methodology and population/sub-population analyses have been previously published. In brief, this community-based cohort was prospectively assembled between 1991 and 1992, from seven townships in Taiwan. Out of a posible 89 293 residents invited, 23 820 (19 665 HBsAg- and 4155 HBsAg+) volunteered for the study. Each provided an informed consent; at enrollment, everyone had the following tests/examinations: abdominal ultrasonography; serologic tests on HBsAg, HBeAg, antibodies against hepatitis C virus (anti-HCV); and serum levels of ALT and α-fetoprotein (AFP). Those who were HBsAg-seropositive (n = 4155) were followed by health examinations regularly scheduled every 6 months-1 year using abdominal ultrasonography and serologic tests until June 30, 2004.
For a complete discussion of the sample collection, endpoint definition and analytic methods please see the original publications. However, because questions remain regarding the impact of the cirrhosis diagnosis technique on the findings we will describe this here for the convenience of the readers. Cirrhosis was ascertained by performing ultrasound examination using high-resolution, real-time ultrasound scanners (Toshibee SSA-240A, Toshiba Co., Tokyo, Japan) with 3.75-MHz convex transducers, based on a previously validated quantitative scoring system including the features of liver surface (normal, irregular or undulated), liver parenchymal texture (normal, heterogeneous, or coarse), size of intrahepatic blood vessel (normal, obscure, or narrowing) and splenic size (normal or enlarged) [15]. All ultrasound examinations were conducted by gastroenterologists certified as specialized physicians by the Society of Medical Ultrasonography in Taiwan. The data linkage to the National Health Insurance profiles in Taiwan was conducted for the complete ascertainment of cirrhosis cases. Further review of the medical records of the identified cases of HCC and cirrhosis was conducted by gastroenterologists using a standard Case Abstraction Form. Clinical information in the Case Abstraction Form was used to confirm HCC cases according to a modification of the established EASL criteria and to assist in diagnosis of cirrhosis cases.
Key findings of REVEAL
Serum HBV DNA level and HCC risk[16]
After excluding subjects with evidence of concurrent infection with the hepatitis C virus or inadequate baseline serum sample for HBV DNA assay, 3653 subjects were included in the HCC outcomes analyses. Around 15% of study participants were HBeAg seropositive; HBeAg-seropositive participants had significantly higher serum HBV DNA levels than HBeAg-seronegative participants (P < 0.001). After 11.4 years and 41 779 person years of follow-up, there were 164 incident HCC cases, the incidence rate of HCC (per 100 000 person-years) increased with serum HBV DNA level at study entry. Compared with participants with study baseline serum HBV DNA levels of <300 copies/ml, the corresponding adjusted hazard ratios (95% CI) increased with serum HBV DNA at study entry (Fig. 1). The importance of serum HBV DNA level as a predictor of HCC was even greater in the subset of HBeAg-seronegative participants with normal ALT levels and no cirrhosis at study entry.
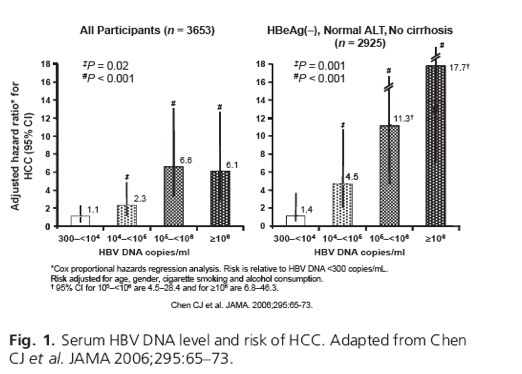
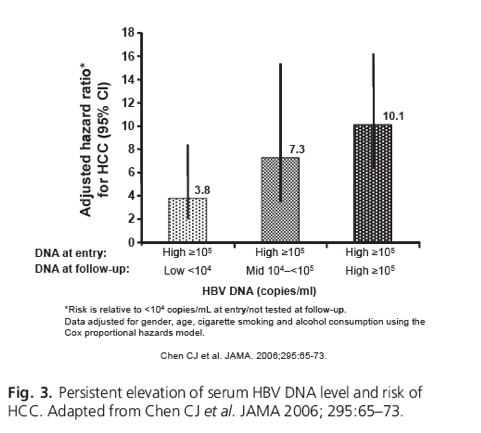
Another key finding of these analyses was the observation that chronic elevation of viral replication carried a higher risk of HCC over the study duration (Fig. 2).
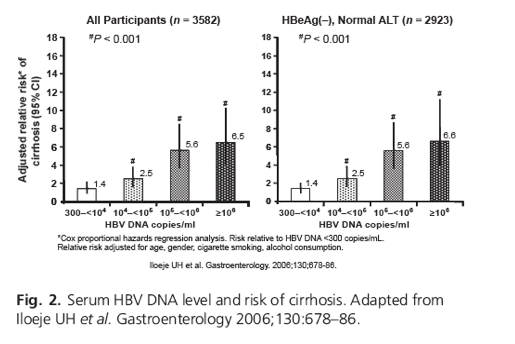
Serum HBV DNA level and risk of cirrhosis[17]
In the analysis of cirrhosis risk, a further 69 participants who had evidence of cirrhosis at study entry, and 2 participants who died from non-liver-related causes within 6 months after enrollment were removed [18]. A total of 3582 participants were retained for the cirrhosis analysis. During 40 038 person-years of follow-up, a total of 365 participants were newly diagnosed with cirrhosis, of which the majority (82%) was confirmed on two or more ultrasound examinations. The incidence rate of cirrhosis (per 100 000 person-years) increased with increasing study entry serum HBV DNA level at study entry. The adjusted hazard ratio (95% CI) increased with entry HBV DNA as well (Fig. 3). Unlike HCC, there was no observed difference in the effect of HBV DNA as a predictor of cirrhosis in the entire population versus the subset of HBeAg- and normal ALT subjects.
Serum HBV DNA level and liver-related mortality[18]
The risk of all-cause and cause-specific mortality and their predictors were also investigated in the REVEAL-HBV study [18]. Of the 23 820 participants enrolled in this prospective cohort, subjects with unknown HCV status (n = 35) or known to be positive for anti-HCV antibody (n = 1313) were removed from the analyses; after 12.5 years and 282 324 person-years of follow-up, there were 1814 deaths. Compared with the HBsAg-seronegative group, HBsAg-seropositive persons had a higher risk of mortality from all-causes; liver cancer; and chronic liver disease and cirrhosis but not for non-liver-related mortality. As much as 41, 96 and 82% of deaths from all causes, liver cancer, and chronic liver disease and cirrhosis, respectively, in the HBsAg-seropositive group was attributable to chronic HBV infection. In HBsAg-seropositive participants, the mortality rate (per 100 000 person-years) increased with baseline serum HBV DNA level; in adjusted analyses, serum HBV DNA level was a significant independent predictor of all-cause mortality and liver-related mortality (liver cancer, chronic liver disease and cirrhosis (Fig. 4).
HBV genotype and mutants and the risk of HCC[19]
In addition to quantitative HBV DNA, its qualitative genetic features were associated with HCC risk as well. Baseline blood samples of 2762 REVEAL-HBV cohort members who had detectable serum HBV DNA level at study entry were tested for HBV genotype [19]. Blood samples of the subset with baseline HBV DNA level ≥ 10 000 copies/ml (n = 1526) were further tested for precore G1896A mutant and basal core promoter (BCP) A1762T/G1764A double mutant. Those with the precore G1896 (wild-type) variant had a higher HCC incidence than for those with the G1896A variant (956 vs.269 per 100 000 person-years); and those with the BCP A1762T/G1764A double mutant had a higher HCC incidence than those with BCP A1762/G1764 (wild-type) variant (1149 vs. 359 per 100 000 person-years). The adjusted hazard ratio (95% CI) of developing HCC was 1.76 (1.19-2.61) for genotype C vs. genotype B; 0.34 (0.21-0.57) for precore G1896A vs. wild-type; and 1.73 (1.13-2.67) for BCP A1762T/G1764A vs. wild-type; serum HBV DNA level remained an independent predictor of HCC.
HCC risk prediction nomograms[20]
From previous studies, several important environmental, host and viral factors predicting risk of developing HCC for patients with chronic hepatitis B have been identified [13, 16, 19]. These risk predictors include age, gender, family history of HCC (host); alcohol consumption (environment); HBeAg serostatus, serum HBV DNA level and HBV genotype (virus); and serum ALT level (interaction between host and virus). Data from the REVEAL-HBV study were used to develop easy-to-use nomograms based on noninvasive clinical parameters for the prediction of HCC in patients with chronic hepatitis B [20]. Three models of varying complexities were derived using Cox regression models; each had gender, age, family history of HCC and serum ALT level as basic risk predictors.
Subsequently, different measures of viral replication status were included as follows: Model 1 included the addition of HBeAg status; Model 2 included different combinations of HBeAg serostatus and quantitative serum HBV DNA level; Model 3 added HBV genotype to the variables in Model 2. Nomograms along with the risk scores were then developed to predict 5- and 10-year HCC risk (nomogram of Model 2 is shown in Fig. 5 as an example). These nomograms were internally validated with respect to discrimination and calibration. All areas under receiver operating curves (AUROCs) were greater than 82%; all correlation coefficients between observed HCC risk and predicted risk were >0.9.
Simplification and external validation of the REVEAL nomogram: REACH-B risk score[21]
Besides the REVEAL-HBV derived HCC risk nomogram, other HCC prediction tools have now been developed and published; however, most were validated using data from the same cohort or from cohorts with similar characteristics in derivation and validation sets [22, 23, 24]. In Addition, most of these other tools were derived from hospital-based cohort studies with smaller sample sizes. Although the model derivation and validation sets used in deriving the REVEAL-HBV HCC risk prediction nomogram were different [20], there remained a need for external validation in a non-Taiwanese population; this resulted in the development of the REACH-B risk score [21].
The REACH-B risk score was based on 3584 REVEAL-HBV Study participants without cirrhosis; the 'validation cohort' was a composite international cohort consisting of 1505 patients from three university hospitals in Hong Kong and Korea. When the risk score was applied to the 'validation cohort', the AUROC (95% CI) for HCC prediction was 0.811 (0.790-0.831) at 3 years, 0.796 (0.775-0.816) at 5 years and 0.769 (0.747-0.790) at 10 years. The tool performed even better after excluding 277 cirrhosis patients in the validation cohort showing the AUROC (95% CI) of 0.902 (0.884-0.918), 0.783 (0.759-0.806) and 0.806 (0.783-0.828), respectively, at 3, 5 and 10 years. Predicted risk calibrated well with Kaplan-Meier observed HCC risk. As this risk score was developed and validated only in Asian patients, the application of this tool to other circumstances, such as other ethnic origins or genetic background, different age at infection, varied HBV genotype of species, as well as additional environmental exposures, should be done with caution. Additional validation studies focused on these diversities will provide further support for its robustness, applicability and transportability.
Incidence and determinants of spontaneous HBsAg seroclearance[25]
The seroclearance of HBsAg has been considered the holy grail of chronic hepatitis B therapy. Persons who become HBsAg-seronegative can be regarded as having resolved their hepatitis B infection [26]. Some CHB patients may spontaneously clear HBsAg, which usually confers a good prognosis if there is no pre-existing HCC or cirrhosis at the time of HBsAg seroclearance [27, 28, 29]. The incidence and determinants of spontaneous HBsAg seroclearance were investigated using the REVEAL-HBV cohort with 3087 participants with chronic HBV infection [25]. HBsAg seroclearance occurred at an annual rate of 2.26%; serum HBV DNA levels at baseline and follow-up examinations were the most significant predictors of HBsAg seroclearance.
Some key takeaways from these analyses are as follows (i) a substantial drop in HBV DNA defined as ≥3 logs is associated with a 4-fold greater likelihood of HBsAg seroclearance compared with those who had a lower drop; (ii) Among subjects that cleared sAg, there was a lag of 4.6 year between the first measured significant drop in HBV DNA and HBsAg seroclearance; (iii) for the few subjects that came in with HBV DNA ≥ 10 000 copies/ml (2000 IU) and were able to totally suppress HBV DNA (<300 copies/ml) before HBsAg seroclearance or censoring, there was a very high rate of HBsAg seroclearance (25.8% at 60 months and 51.3% at 100 months; slope 0.5% per month). Another interesting observation was that having a mildly elevated ALT at the time of complete suppression of HBV DNA was associated with a greater likelihood of clearing the HBsAg; potentially an indication of an active immune system. These findings are consistent with previously published literature showing that within randomized clinical trials, subjects with post-treatment HBV-DNA level of <1000 copies/ml had a higher likelihood of achieving HBsAg loss [30]. These analyses not only indicate that HBsAg seroclearance in hyper-endemic areas is not as rare as previously thought, but also provide insights into potential design features for future clinical trials seeking to evaluate HBsAg clearance as an endpoint.
HCC incidence and liver-related mortality in inactive HBV carriers[31]
In the natural history of chronic HBV infection, an inactive HBV carrier state (presence of HBsAg but not HBeAg in serum, no ALT elevations and serum HBV DNA levels <10 000 copies/ml) is presumed to carry a very low risk of liver disease progression [32]. The risk of HCC development and death from liver-related causes was compared between 1932 inactive HBV carriers and 18 137 participants who were HBsAg-seronegative and anti-HCV-seronegative with normal ALT levels (control group) [31]. Those that had cirrhosis before study entry or developed end-stage liver diseases in the first year of follow-up were excluded from both groups. The annual event rates were very low between the inactive HBV carriers and controls: HCC was 0.06% vs. 0.02%; liver-related death was 0.04% vs. 0.02%. Although the absolute risks are relatively small, the multivariate-adjusted hazard ratios (95% CI) for HCC and liver-related death was 4.6 (2.5-8.3) and 2.1 (1.1-4.1), respectively, for inactive carriers compared with controls.
HBV infection and risk of pancreatic cancer[33]
The hepatitis B virus once thought to be purely hepatotropic, has been detected in several extrahepatic tissues including the pancreas [34]. The REVEAL-HBV cohort was used to investigate the relationship between chronic infection with active replication of HBV and risk of pancreatic cancer [33]. A full description of the methods has been already published in this journal. Pancreatic cancer diagnoses were ascertained through data linkage with the National Cancer Registry and Death Certification System in Taiwan from January 1, 1991 to December 31 2007. A total of 22 471 subjects (3930 HBsAg-seropositive) who were anti-HCV-seronegative and free of pancreatic cancer at entry were part of this analysis. Overall, there were 48 pancreatic cancer cases. The HBsAg-seropositive subjects were further classified by HBeAg and serum HBV DNA level for the analysis of the association between markers of active viral replication and pancreatic cancer. Chronic HBsAg carriers had a 2-fold increased risk of pancreatic cancer after adjustment for gender, age at enrolment, cigarette smoking and alcohol consumption. The subgroup analysis showed that chronic HBV infection was associated with an increased risk of pancreatic cancer most notably in females, individuals ²50 years of age, non-smokers and non-drinkers. An increased risk of pancreatic cancer was observed for HBsAg-seropositives with active HBV replication exclusively. However, the lack of association in some subgroups/categories may be because of small numbers of pancreatic cancer cases, thus the possibility of type II error should be taken into consideration when interpreting these data. Although no causal inference can be made, these results not only support an association between chronic HBV infection with active replication and increased pancreatic cancer risk, but represent the most provocative finding from the REVEAL-HBV study to date.
Revelations, insights and questions from the REVEAL-HBV study
Data from the REVEAL-HBV study have helped establish an HBV viral load paradigm in the natural history of chronic hepatitis B [8, 35, 36, 37]. Serum HBV DNA level was identified as the most important risk predictor for progressing to end-stage liver disease outcomes [17, 18, 38]. A fairly significant finding was the biological gradient of disease progression risk across the serum HBV DNA levels from a single measurement; in the case of progression to HCC, this risk gradient was found to be even more striking among chronic HBV carriers who were HBeAg-seronegative with normal ALT levels.
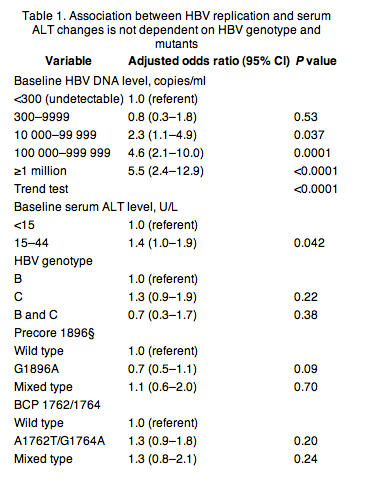
Several questions have been raised by this study; a major one is how a single HBV DNA test can reliably predict the outcomes of cirrhosis, HCC and death many years after? We cannot offer a definitive answer to this question; however we offer some thoughts that may aid in understanding this observation. The first thing to keep in mind is that the subjects entered the cohort as adults and their viral history during childhood and adolescence remains unknown. It is therefore likely that the baseline HBV DNA reflects a long duration of HBV DNA elevation in a subset of patients, a large number of whom have gone through the immune clearance phase (85% were HBeAg-negative) without successfully clearing the virus; these subjects are understood to remain at risk for future hepatic inflammation/fibrosis. Supporting evidence for this comes from subsequent analyses of this cohort; first, in a subsequent time dependent analyses including information on multiple HBV DNA time point, the results observed using the single baseline HBV DNA were confirmed [39]. Secondly, in the analyses of HCC risk as determined by the changes in serum HBV DNA levels over the follow-up period, we observed an association between the HBV DNA trajectory and the changes in serum ALT level over time [40, 41]. We subsequently examined this in a follow-up analysis and observed that male gender and baseline HBV level were the two strongest predictors of future ALT elevation (a surrogate for hepatic inflammation); this association was independent of HBV genotype or precore/BCP mutant status (Table 1) [42]. Our inference therefore is that the study entry single HBV DNA in this population accurately represented what must have been a prolonged period of HBV elevation; this may be particularly more relevant to the patients with childhood HBV infection. Importantly, these data cannot be viewed to directly reflect what will happen in other types of HBV infected populations.
Another key question raised by these observations is, should patients with persistently high HBV DNA be treated regardless of serum ALT level? This is a tempting conclusion and may not be entirely unreasonable given the observation that patients with normal serum ALT could already have significant liver disease [43, 44, 45], however, this is a question that guideline development committees are best suited to answer based upon the best available evidence. It is indeed our perspective that part of the answer to this question depends on other risk factors such as age and gender. A persistently elevated HBV DNA in someone of similar background to the population of REVEAL should prompt a serious consideration for much closer follow-up; if evidence of unexplained ALT elevation is observed, that may be enough for consideration for antiviral therapy if age and gender also put the person at risk. The decision for antiviral therapy must also take into consideration the HBeAg status and the benefit risk profile of the particular antiviral options that are available to the prescriber and patient. It was in an attempt to address this question that we developed the risk stratification nomograms which have also been externally validated in a cohort that is not only different from the REVEAL-HBV cohort in several demographical and disease characteristics (gender, age, HBeAg serostatus, serum ALT and HBV DNA levels and cirrhosis status at study entry), but also in being a hospital based cohort in contrast to a community dwelling cohort. The development of the nomograms has raised the question of how they should be used. It is our recommendation that they be viewed primarily as a risk stratification tool that helps clinicians engage their patients in risk communication; this should then prompt a discussion of who to follow more closely for cancer surveillance. Before any firm recommendation of what score should prompt closer monitoring vs. consideration for treatment, a prospective trial probably should be conducted applying the risk score in a liver cancer surveillance programme to see how it performs and what threshold score should be recommended. Such a trial is beyond the expertise of our study group.
The modality for diagnosing cirrhosis in this cohort has also been an area of questioning; a concern is the potential for underreporting the presence of cirrhosis at baseline as well as during follow up. We have provided a full description of the quantitative scoring algorithm used for the cirrhosis diagnosis; this is a validated algorithm. Although ultrasound as a modality for diagnosing cirrhosis may not be the gold standard, utilizing a structured scoring algorithm as was used in our study has been shown to have a sensitivity and specificity ≥80% as reviewed in our primary paper on cirrhosis risk [17]. We are fairly confident that the false negative rate though unlikely to be zero, is fairly low. In addition, we attempted to account for the risk of false positives in our original article by restricting the case definition of cirrhosis in a sensitivity analysis to those with at least 2 concurring ultrasound tests; in that analysis the HRadj for cirrhosis development associated with serum HBV DNA level was even greater. This suggests that the bias in our results was towards underestimating the impact of HBV DNA on cirrhosis risk and not in the other direction. Finally, because none of the participants had a confirming liver biopsy done, this question will predictably remain a limitation of this dataset; however it is not a limitation likely to nullify the observations as reported.
The level of circulating HBV DNA predicted liver-related outcomes even accounting for HBV genotype; it also predicted the serological outcome of HBsAg seroclearance. The observed association between low levels of HBV DNA and eventual cumulative loss of HBsAg in the absence of antiviral therapy has not previously been described in any study of this nature and size. This finding is consistent with, and provides more detailed information in support of Kim et al. who found in a small group of Korean patients (N = 215) followed for 48 months that HBsAg clearance occurred exclusively in subjects who were HBeAg negative with undetectable HBV DNA [46]. Subsequent to our publication, others have indicated that among subjects with low level HBV DNA, the HBsAg titre predicts HBsAg loss [47]. We view this as consistent with our finding above; furthermore we have also reported from REVEAL that the HBsAg titer and HBV DNA levels are correlated [48].
The real lesson from REVEAL is not that serum HBV viral load is an important predictor of liver-related disease outcomes in chronic hepatitis B, this in of itself was not entirely novel. The tightness of the correlation, its temporal nature and the biological gradient of this association was a key finding. This study helps connect the dots in the natural history of chronic hepatitis B in a population of untreated subjects like no other cohort was able to do before it.
A relatively novel contribution from this large scale study that warrants further investigation is the association between chronic infection of HBV especially in active replication and an increased risk of pancreatic cancer [33]. There is evidence in the literature showing the presence of HBV viral particles in the pancreas with a potential association with pancreatic inflammation [49, 50]. As far as we can tell, there is little clarity on how chronic HBV infection may result in pancreatic carcinoma. A possible theory is the potential role of HBV-related acute/chronic inflammation of the pancreas on pancreatic carcinogenesis. This is supported by reports of elevation of pancreatic enzymes in chronic HBV infection[51] and acute viral hepatitis [52]. Although there is less evidence for this, there may be integration of the HBV viral particles into the host genome similar to what has been described for HBV-related HCC. It is known that in chronic HBV infected persons, the virus is present within pancreatic cells [53, 54]. More needs to be done to explore this potential association not only in Asians with chronic HBV infection but in non-Asians as well; if confirmatory evidence is found, it may provide an opportunity to screen these patients for this very deadly cancer.
Taking all the evidence into account, the amount of circulating serum HBV DNA does play a central role in the disease progression in patients with chronic hepatitis B, as well as in the transition across the different stages in the natural history. Besides helping elucidate further the natural history of chronic HBV infection, the findings of the REVEAL-HBV study also provide some foundational information to support an approach of maximal and timely suppression of viral replication as the target of therapy in the management patients with chronic hepatitis B. These data indicate that such an approach should lower the risk of disease progression and increase the probability of reaching the ultimate goal of therapy, HBsAg seroclearance/seroconversion.
The REVEAL-HBV study will surely continue to extend our knowledge of the natural history of chronic hepatitis B. Further analyses of this dataset will focus on the delineation of the probabilities and determinants of seroclearance of HBeAg and serum HBV DNA; as well as the liver diseases progression according to the changes in HBeAg status, serum HBV DNA and ALT levels and HBsAg serostatus.
The quantification of HBsAg in serum is an area of current research interest.
Recent evidence has shown that both HBsAg and HBV DNA levels decline along the natural course of chronic HBV infection [55]. The kinetics of HBsAg decline in the natural history and its independent/interactive role with existing factors for the development of adverse outcomes are areas of top priority. Research on host genetic variants associated with the development of HCC and cirrhosis, and associated with virus-related seromarkers and their change over time are also areas of continuing interest [56, 57]. Addressing these and other questions yet to be determined should contribute not only to the science of chronic hepatitis B disease progression but also to making the clinical management of patients more successful.
|
|
| |
| |
|
|
|